| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
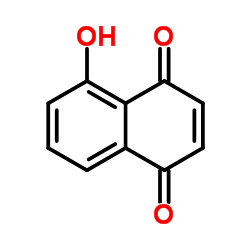 |
胡桃酮,5-羟基对萘醌
CAS:481-39-0 |
|
 |
黄钟花醌
CAS:84-79-7 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
胡桃酮,5-羟基对萘醌
CAS:481-39-0 |
|
 |
黄钟花醌
CAS:84-79-7 |