A highly active chemotactic peptide analog incorporating the unusual residue 1-aminocyclohexanecarboxylic acid at position 2.
M Sukumar, P A Raj, P Balaram, E L Becker
文献索引:Biochem. Biophys. Res. Commun. 128(1) , 339-44, (1985)
全文:HTML全文
摘要
Analogs of chemotactic peptides (Formyl-Met-X-Phe-OMe) containing the stereochemically constrained residues alpha-aminoisobutyric acid (Aib), 1-aminocyclopentanecarboxylic acid (Acc5) and 1-aminocyclohexanecarboxylic acid (Acc6) at position 2 are compared with the parent sequence (X = Leu) for their ability to induce lysozyme release in rabbit neutrophils. The Acc6 analog is about 78 times more active than the parent peptide, For-Met-Leu-Phe-OH, whereas Aib and Acc5 analogs are approximately 3 and 2 times, respectively, less active than the parent peptide. NMR and model building studies clearly favour a Met-Acc6 beta-turn solution conformation in the Acc6 analog, suggesting that the neutrophil receptor is capable of recognizing a folded peptide structure. The significant differences in the activities of the Acc5 and Acc6 analogs suggest an important role for the residue 2 sidechain in receptor interactions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
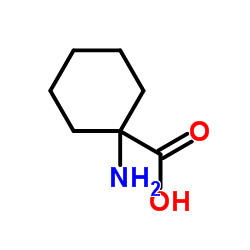 |
1-氨基-1-环己基甲酸
CAS:2756-85-6 |
C7H13NO2 |
|
Drug design, in vitro pharmacology, and structure-activity r...
2009-08-27 [J. Med. Chem. 52 , 5093-107, (2009)] |
|
Application of 1-aminocyclohexane carboxylic acid to protein...
2008-02-01 [J. Chem. Inf. Model. 48(2) , 333-43, (2008)] |
|
Influence of 1-aminocyclohexane-1-carboxylic acid in positio...
2003-08-01 [J. Pept. Res. 62(2) , 70-7, (2003)] |
|
Highly potent 1-aminocyclohexane-1-carboxylic acid substitut...
2004-11-18 [J. Med. Chem. 47(24) , 6020-4, (2004)] |
|
Nitroxyl peptides as catalysts of enantioselective oxidation...
2002-01-04 [Chemistry 8(1) , 84-93, (2002)] |