| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氧化亚铁
CAS:1345-25-1 |
|
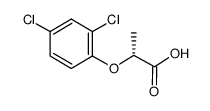 |
(R)-2-(2,4-二氯苯氧基)丙酸
CAS:15165-67-0 |
|
 |
2,4-滴丙酸
CAS:120-36-5 |