Purification and characterization of chitinase from Paenibacillus sp. D1.
Anil Kumar Singh, Hari S Chhatpar
文献索引:Appl. Biochem. Biotechnol. 164(1) , 77-88, (2011)
全文:HTML全文
摘要
A 56.56-kDa extracellular chitinase from Paenibacillus sp. D1 was purified to 52.3-fold by ion exchange chromatography using SP Sepharose. Maximum enzyme activity was recorded at pH 5.0 and 50 °C. MALDI-LC-MS/MS analysis identified the purified enzyme as chitinase with 60% similarity to chitinase Chi55 of Paenibacillus ehimensis. The activation energy (E (a)) for chitin hydrolysis and temperature quotient (Q (10)) at optimum temperature was found to be 19.14 kJ/mol and 1.25, respectively. Determination of kinetic constants k (m), V (max), k (cat), and k (cat)/k (m) and thermodynamic parameters ΔH*, ΔS*, ΔG*, ΔG*(E-S), and ΔG*(E-T) revealed high affinity of the enzyme for chitin. The enzyme exhibited higher stability in presence of commonly used protectant fungicides Captan, Carbendazim, and Mancozeb compared to control as reflected from the t (1/2) values suggesting its applicability in integrated pest management for control of soil-borne fungal phytopathogens. The order of stability of chitinase in presence of fungicides at 80 °C as revealed from t (1/2) values and thermodynamic parameters E (a(d)) (activation energy for irreversible deactivation), ΔH*, ΔG*, and ΔS* was: Captan > Carbendazim > Mancozeb > control. The present study is the first report on thermodynamic and kinetic characterization of chitinase from Paenibacillus sp. D1.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
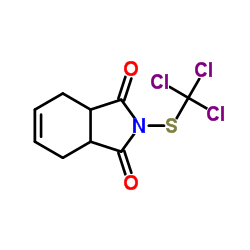 |
克菌丹
CAS:133-06-2 |
C9H8Cl3NO2S |
|
Determination of pesticides in sunflower seeds by high-perfo...
2014-01-01 [J. AOAC Int. 97(4) , 1012-20, (2014)] |
|
[Survey of pesticide residues in imported frozen vegetables ...
2011-01-01 [Shokuhin Eiseigaku Zasshi 52(2) , 121-9, (2011)] |
|
Toxicokinetics of captan and folpet biomarkers in orally exp...
2012-03-01 [J. Appl. Toxicol. 32(3) , 194-201, (2012)] |
|
Air pollutants formed in thermal decomposition of folpet fun...
2011-01-15 [Environ. Sci. Technol. 45(2) , 554-60, (2011)] |
|
Effects of captan on Apis mellifera brood development under ...
2009-02-01 [J. Econ. Entomol. 102(1) , 20-9, (2009)] |