| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
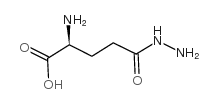 |
L-谷氨酸=gamma-肼
CAS:1820-73-1 |
|
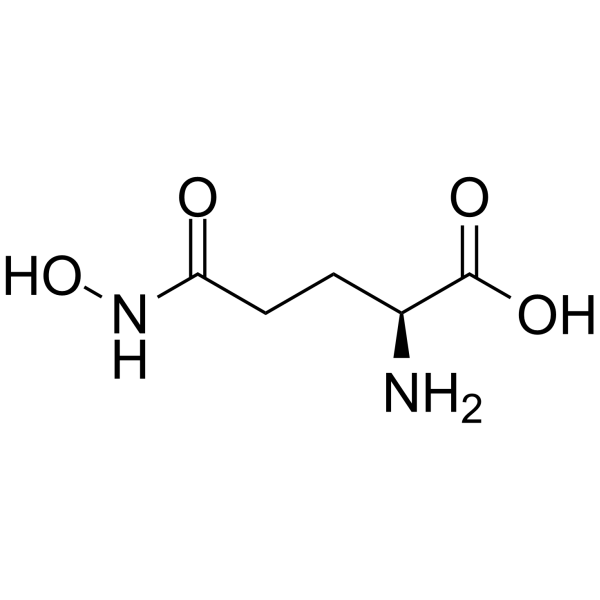 |
L-Glutamic acid γ-monohydroxamate
CAS:1955-67-5 |