Development of plasma kallikrein selective inhibitors.
Y Okada, Y Tsuda, M Tada, K Wanaka, A Hijikata-Okunomiya, U Okamoto, S Okamoto
文献索引:Biopolymers 51(1) , 41-50, (1999)
全文:HTML全文
摘要
During the course of the development of active center-directed plasmin inhibitors, it was found that N-(trans-4-aminomethylcyclohexanecarbonyl)-lysine-4-methoxycarb onylanilide inhibited plasma kallikrein more potently than other enzymes such as plasmin, urokinase, and thrombin, although the inhibitory activity was not as potent and enzyme selectivity not as high. Based on studies of structure-activity relationship, we designed and synthesized the plasma kallikrein selective inhibitor, N-(trans-4-aminomethylcyclohexanecarbonyl)-phenylalanine-4-carboxy methyl- anilide (Tra-Phe-APAA). Tra-Phe-APAA inhibited plasma kallikrein with a Ki value of 0.81 microM, while it inhibited glandular kallikrein, plasmin, urokinase, tissue plasminogen activator, factor Xa, factor XIIa, and thrombin with Ki values of > 500, 390, 200, > 500, > 500 > 500, and > 500 microM, respectively. We designated Tra-Phe-APAA as PKSI-527. Using PKSI-527 as an affinity ligand, we synthesized a new affinity gel (PKSI-Toyopearl) and employed it for the rapid purification of plasma kallikrein from human plasma. Human plasma activated with kaolin after acid treatment was applied to a PKSI-527-Toyopearl column. Adsorbed protein was eluted with 50 mM glycinehydrochloric acid buffer (pH 3.0). Plasma kallikrein was purified 181-fold with a yield of 85% from the kaolin-activated plasma.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
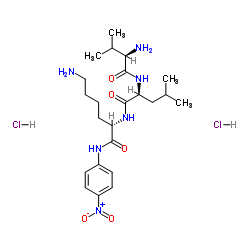 |
D-缬氨酰-L-亮氨酰-L-赖氨酰-对-硝基苯胺二盐酸盐
CAS:62354-43-2 |
C23H40Cl2N6O5 |
|
The potential mechanism for the effect of heparin on tissue ...
2000-03-01 [Thromb. Res. 97(5) , 349-58, (2000)] |
|
Bovine erythrocyte haemolysates enhance plasminogen activati...
1997-02-01 [Vet. Res. Commun. 21(2) , 75-84, (1997)] |
|
Gossypol-induced inhibition of plasminogen activator activit...
1999-12-01 [Andrologie 31(6) , 355-9, (1999)] |
|
Activating effect of the plasminogen activators on plasminog...
1995-08-15 [Thromb. Res. 79(4) , 423-8, (1995)] |
|
A novel assay to measure plasminogen activation capacity in ...
1994-06-15 [Thromb. Res. 74(6) , 665-72, (1994)] |