| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
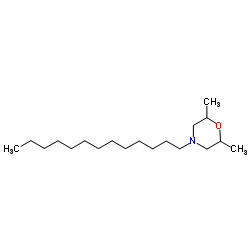 |
异十三吗啉
CAS:24602-86-6 |
|
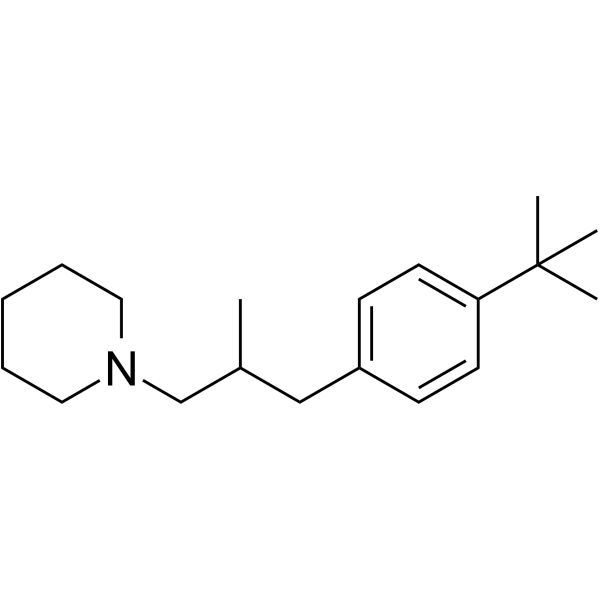 |
苯锈啶
CAS:67306-00-7 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
异十三吗啉
CAS:24602-86-6 |
|
 |
苯锈啶
CAS:67306-00-7 |