| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
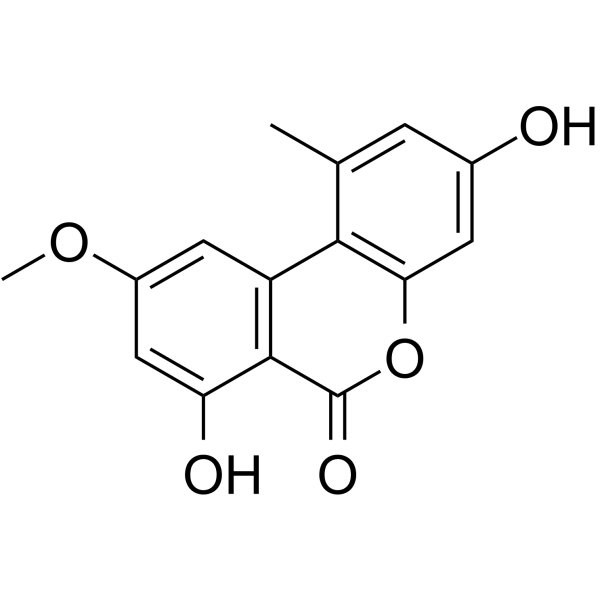 |
交链孢酚单甲醚
CAS:23452-05-3 |
|
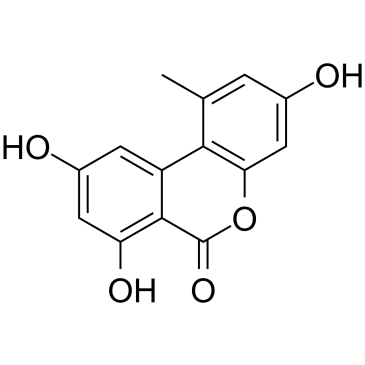 |
交链孢酚
CAS:641-38-3 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
交链孢酚单甲醚
CAS:23452-05-3 |
|
 |
交链孢酚
CAS:641-38-3 |