Adsorption of Acid Red 73 on copper dithiocarbamate precipitate-type solid wastes.
Fenglian Fu, Ya Xiong, Baoping Xie, Runming Chen
文献索引:Chemosphere 66(1) , 1-7, (2007)
全文:HTML全文
摘要
Three solid wastes, copper N,N'-bis(dithiocarboxy)piperazine ([CuBDP](n)), copper diethyldithiocarbamate (Cu(DDTC)(2)) and copper dimethyldithiocarbamate (Cu(DMTC)(2)), were prepared and tested as adsorbents to remove Acid Red 73 from wastewater. It was found that the three precipitates all could effectively adsorb the dye but their adsorption abilities were rather different. The maximum adsorption amounts of the coordination polymer precipitate [CuBDP](n) reached as high as 364mg g(-1), much greater than those of Cu(DDTC)(2) and Cu(DMTC)(2) (42.9 and 37.8mg g(-1), respectively). The investigation of adsorption models showed these adsorption processes followed the pseudo-second-order kinetic equation and the adsorption balances could be described with both Langmuir and Freundlich isotherms, but the latter seemed to be more suitable. Their adsorption nature was inferred to be physical adsorption and mainly depended on the hydrophobic interaction between these precipitates and Acid Red 73. This is the first example for the reutilization of metal dithiocarbamate precipitates as solid wastes to date.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
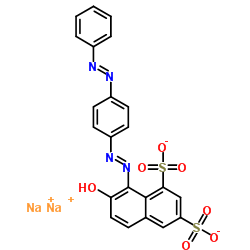 |
酸性红73
CAS:5413-75-2 |
C22H14N4Na2O7S2 |
|
A single-step simultaneous protein staining procedure for po...
2012-01-01 [Methods Mol. Biol. 869 , 551-9, (2012)] |
|
Glow discharge plasma in water: a green approach to enhancin...
2012-01-30 [J. Hazard. Mater. 201-202 , 162-9, (2012)] |
|
Efficient removal of dyes in water using chitosan microspher...
2010-05-15 [J. Hazard. Mater. 177(1-3) , 560-6, (2010)] |
|
The azo dye solvent yellow 3 produces depigmentation.
2000-04-01 [Contact Dermatitis 42(4) , 237-8, (2000)] |
|
Single-beam Z-scan measurement of the third-order optical no...
2007-11-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 68(3) , 578-82, (2007)] |