Transient state kinetic investigation of 5-aminolevulinate synthase reaction mechanism.
Junshun Zhang, Gloria C Ferreira
文献索引:J. Biol. Chem. 277(47) , 44660-9, (2002)
全文:HTML全文
摘要
5-Aminolevulinate synthase (ALAS), a pyridoxal 5'-phosphate-dependent enzyme, catalyzes the first, and regulatory, step of the heme biosynthetic pathway in nonplant eukaryotes and some bacteria. 5-Aminolevulinate synthase is a dimeric protein having an ordered kinetic mechanism with glycine binding before succinyl-CoA and with aminolevulinate release after CoA and carbon dioxide. Rapid scanning stopped-flow absorption spectrophotometry in conjunction with multiple turnover chemical quenched-flow kinetic analyses and a newly developed CoA detection method were used to examine the ALAS catalytic reaction and identify the rate-determining step. The reaction of glycine with ALAS follows a three-step kinetic process, ascribed to the formation of the Michaelis complex and the pyridoxal 5'-phosphate-glycine aldimine, followed by the abstraction of the glycine pro-R proton from the external aldimine. Significantly, the rate associated with this third step (k(3) = 0.002 s(-1)) is consistent with the rate determined for the ALAS-catalyzed removal of tritium from [2-(3)H(2)]glycine. Succinyl-CoA and acetoacetyl-CoA increased the rate of glycine proton removal approximately 250,000- and 10-fold, respectively, supporting our previous proposal that the physiological substrate, succinyl-CoA, promotes a protein conformational change, which accelerates the conversion of the external aldimine into the initial quinonoid intermediate (Hunter, G. A., and Ferreira, G. C. (1999) J. Biol. Chem. 274, 12222-12228). Rapid scanning stopped-flow and quenched-flow kinetic analyses of the ALAS reaction under single turnover conditions lend evidence for two quinonoid reaction intermediates and a model of the ALAS kinetic mechanism in which product release is at least the partially rate-limiting step. Finally, the carbonyl and carboxylate groups of 5-aminolevulinate play a major protein-interacting role by inducing a conformational change in ALAS and, thus, possibly modulating product release.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
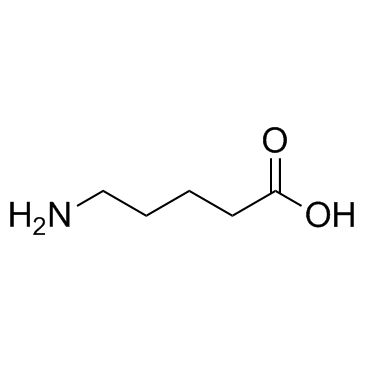 |
5-氨基戊酸
CAS:660-88-8 |
C5H11NO2 | |
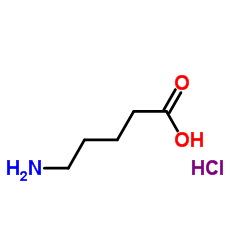 |
5-氨基戊酸盐酸盐
CAS:627-95-2 |
C5H12ClNO2 |
|
Peptide dimethylation: fragmentation control via distancing ...
2014-10-01 [J. Am. Soc. Mass Spectrom. 25(10) , 1694-704, (2014)] |
|
Detection of autosomal dominant polycystic kidney disease by...
2011-06-01 [Kidney Int. 79(11) , 1244-53, (2011)] |
|
Highly sensitive GC/MS/MS method for quantitation of amino a...
2011-04-01 [Anal. Chem. 83(7) , 2705-11, (2011)] |
|
Differential 12C-/13C-isotope dansylation labeling and fast ...
2009-05-15 [Anal. Chem. 81(10) , 3919-32, (2009)] |
|
Development of imidazole alkanoic acids as mGAT3 selective G...
2011-05-01 [Eur. J. Med. Chem. 46 , 1483-98, (2011)] |