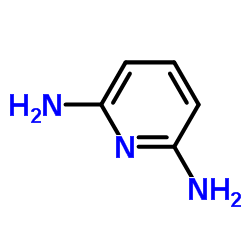
|
~% |
|
~90% |
|
~% |
|
~% |
|
~57% |
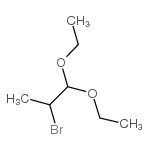

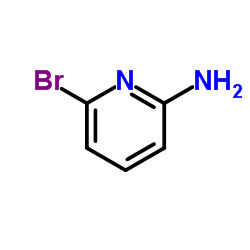
![5-溴-3-甲基咪唑[1,2-a]吡啶结构式](https://image.chemsrc.com/caspic/463/4926-54-9.png)
![3-甲基-5-氨基咪唑并[1,2-a]吡啶结构式](https://image.chemsrc.com/caspic/235/74420-50-1.png)