Reversed-phase liquid chromatographic determination of tramiprosate in rat plasma using evaporative light scattering detector.
R Nageswara Rao, Pawan K Maurya, Dhananjay D Shinde, Sachin B Agwane
文献索引:Biomed. Chromatogr. 25(8) , 925-9, (2011)
全文:HTML全文
摘要
A simple and reliable method was developed for determining blood concentrations of tramiprosate using reversed-phase HPLC with evaporative light scattering detection. The optimum evaporator and nebulizer temperatures for ELSD were set at 90 and 60°C respectively. The method was linear for a concentration range of 10-1000 µg/mL when 0.3 mL blood was used. The intra-assay precision ranged from 1.84 to 5.24%, and the coefficient of variation (CV) for a quality control sample at 10 µg/mL was 5.24% with a bias of -9.50% from the target value. The inter-assay precision ranged from 2.58 to 5.96%, and the CV for a quality control sample at 10 µg/mL was 5.96% with a bias of -7.50% from the target value. The method described here is suitable for management of tramiprosate in Alzheimer disease therapy.Copyright © 2010 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
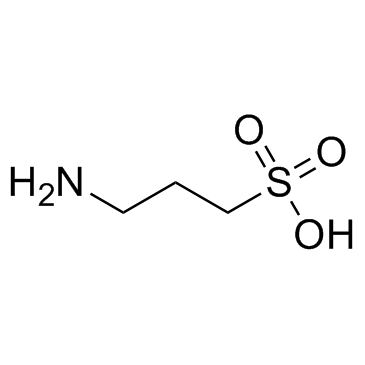 |
高牛磺酸
CAS:3687-18-1 |
C3H9NO3S |
|
Altering in vivo macrophage responses with modified polymer ...
2015-07-01 [Biomaterials 56 , 187-97, (2015)] |
|
Phospho-iTRAQ: assessing isobaric labels for the large-scale...
2015-02-06 [J. Proteome Res. 14(2) , 839-49, (2015)] |
|
Effect of bore fluid composition on microstructure and perfo...
2015-05-15 [J. Chromatogr. A. 1394 , 148-53, (2015)] |
|
Modulating bilayer mechanical properties to promote the coup...
2015-10-01 [Eur. Biophys. J. 44 , 503-12, (2015)] |
|
Role of K+ -channels in homotaurine-induced analgesia.
2001-06-01 [Fundam. Clin. Pharmacol. 15(3) , 167-73, (2001)] |