| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
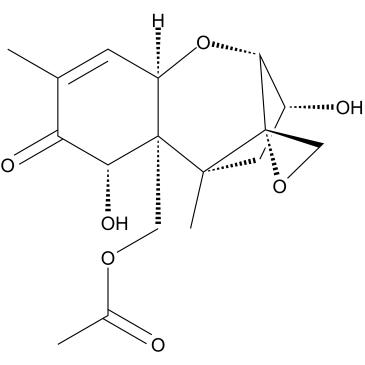 |
15-O-乙酰脱氧瓜萎镰菌醇
CAS:88337-96-6 |
|
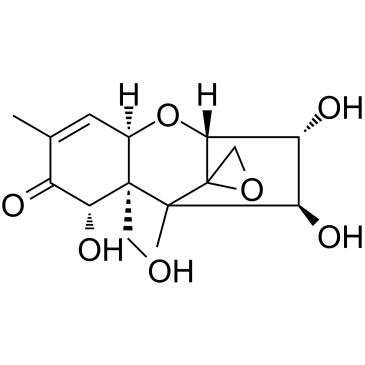 |
瓜萎镰菌醇
CAS:23282-20-4 |
|
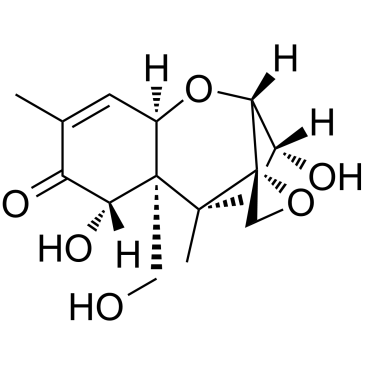 |
脱氧瓜萎镰菌醇
CAS:51481-10-8 |
|
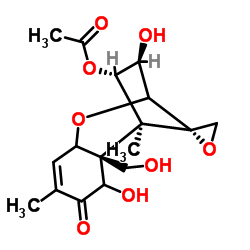 |
镰刀菌烯酮
CAS:23255-69-8 |