Enantioselective synthesis and olfactory evaluation of 13-alkyl-substituted alpha-ionones.
Marco Luparia, Paolo Boschetti, Francesca Piccinini, Alessio Porta, Giuseppe Zanoni, Giovanni Vidari
文献索引:Chem. Biodivers. 5(6) , 1045-57, (2008)
全文:HTML全文
摘要
To study the influence of the steric bulk of the substituents at C(5) on the olfactory characteristics of alpha-ionone, the (S)-antipodes of compounds 8-10 were synthesized starting from (S)-alpha-cyclogeraniol (14a). The latter was available in useful preparative yield with 95% ee by enantioselective lipase-PS-mediated acetylation of the racemic mixture. Key step in the conversion of 14a to 8-10 was an S(N)2'-type reaction of an organocuprate on the allylic phosphate 20, which appears to be a general method for the introduction of an alkyl substituent at the cyclohexene C=C bond of ionones. Olfactory evaluation showed that, compared to the parent (S)-alpha-ionone (1), the odor strength and fragrance facets of the three analogues 8-10 are significantly influenced by the bulkiness of the substituent at C(13), giving further evidence that hydrophobic interactions of this group play a significant role in the chemoreception of ionones. In particular, the odor of the ethyl derivative 8 was found to be significantly stronger than that of the parent (S)-alpha-ionone (1).
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
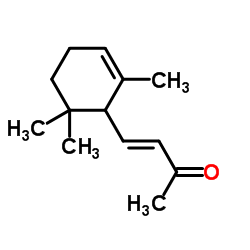 |
紫罗兰酮
CAS:127-41-3 |
C13H20O |
|
Biosynthesis of α- and β-ionone, prominent scent compounds, ...
2012-01-01 [Acta Biochim. Pol. 59(1) , 79-81, (2012)] |
|
CYP264B1 from Sorangium cellulosum So ce56: a fascinating no...
2012-07-01 [Appl. Microbiol. Biotechnol. 95(1) , 123-33, (2012)] |
|
New attractants for males of the solanaceous fruit fly Bactr...
2008-12-01 [J. Chem. Ecol. 34(12) , 1532-5, (2008)] |
|
Stereo- and regioselective hydroxylation of alpha-ionone by ...
1998-10-01 [Appl. Environ. Microbiol. 64(10) , 3878-81, (1998)] |
|
Enhancement of attraction of alpha-ionol to male Bactrocera ...
2001-02-01 [J. Econ. Entomol. 94(1) , 39-46, (2001)] |