Journal of Medicinal Chemistry
1976-02-01
Cycloalkanones. 8. Hypocholesterolemic activity of long-chain ketones related to pentadecanone.
S D Wyrick, I H Hall, C Piantadosi, C R Fenske
文献索引:J. Med. Chem. 19(2) , 219-22, (1976)
全文:HTML全文
摘要
Aliphatic analogs of 2,8-dibenzylcyclooctanone which includes C15-C18 ketones have been investigated for hypocholesterolemic activity in rats. The position of the carbonyl group in the chain for maximum activity appears to be the 2 position. 2-Hexadecanone reduced serum cholesterol levels significantly without altering serum triglyceride levels. This drug was not estrogenic at effective doses which is in contrast to the cyclooctanones which possess this activity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
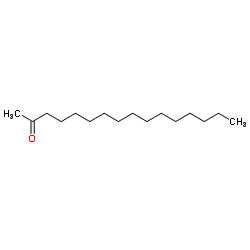 |
2-十六烷酮
CAS:18787-63-8 |
C16H32O |
相关文献:
更多...
|
Identification of the female-produced sex pheromone of the s...
2003-07-01 [J. Chem. Ecol. 29(7) , 1635-42, (2003)] |
|
A glucose/hydrogen peroxide biofuel cell that uses oxidase a...
2002-05-15 [Bioelectrochemistry 56(1-2) , 99-105, (2002)] |
|
Cycloalkanones. 9. Comparison of analogues which inhibit cho...
1976-10-01 [J. Med. Chem. 19(10) , 1257-61, (1976)] |
|
Lipids in the femoral gland secretions of male Schreiber's g...
2006-01-01 [Z. Naturforsch., C, J. Biosci. 61(9-10) , 763-8, (2006)] |
|
Amperometric pH-sensing biosensors for urea, penicillin, and...
[Anal. Chim. Acta 415(1) , 151-157, (2000)] |