The synthesis of androstane brassinosteroid analogues with alpha-azido acid ester groups in position 17beta.
Jaroslava Hnilickova, Ladislav Kohout, Enric Capdevila, Ana Esteve, Marc Vilaplana, Meritxell Molist, Carme Brosa, Jana Swaczynova-Oklestkova, Barbora Slavikova
文献索引:Steroids 75(12) , 1005-10, (2010)
全文:HTML全文
摘要
Androstane brassinosteroid analogues with alpha-azido acid ester groups in position 17beta were synthesized from 2alpha,3alpha,17beta-trihydroxy-5alpha-androstan-6-one after the protection of the 2alpha,3alpha-diols upon treatment with the corresponding alpha-azido acid and the subsequent deprotection of the diol grouping. Six new castasterone analogues were prepared. The biological activities were evaluated in two bioassays: a rice lamina inclination test and bean second internode bioassays. The activities of the new analogues differ in these two bioassays.Copyright 2010 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
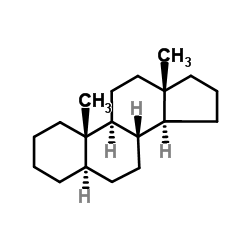 |
5-ALPHA-雄烷
CAS:438-22-2 |
C19H32 |
|
Potent and selective steroidal inhibitors of 17beta-hydroxys...
2009-12-10 [J. Med. Chem. 52 , 7488-502, (2009)] |
|
Novel and efficient synthesis and antifungal evaluation of 2...
2008-01-01 [Bioorg. Med. Chem. Lett. 20 , 7372-5, (2010)] |
|
[Bioconversion of C19- and C21-steroids with parent and muta...
2010-01-01 [Prikl. Biokhim. Mikrobiol. 46(2) , 212-20, (2010)] |
|
Synthesis, antiproliferative activity, acute toxicity and as...
2011-07-01 [Arch. Pharm. Res. 34(7) , 1055-63, (2011)] |
|
Synthesis, characterization and biological evaluation of som...
2011-01-01 [Steroids 76(7) , 709-23, (2011)] |