Additive transfer free energies of the peptide backbone unit that are independent of the model compound and the choice of concentration scale.
Matthew Auton, D Wayne Bolen
文献索引:Biochemistry 43(5) , 1329-42, (2004)
全文:HTML全文
摘要
With knowledge of individual transfer free energies of chemical groups that become newly exposed on protein denaturation and assuming the group transfer free energy contributions are additive, it should be possible to predict the stability of a protein in the presence of denaturant. Unfortunately, several unresolved issues have seriously hampered quantitative development of this transfer model for protein folding/unfolding. These issues include the lack of adequate demonstration that group transfer free energies (DeltaG(tr)) are additive and independent of the choice of model compound, the problem arising from dependence of DeltaG(tr) on concentration scales, the lack of knowledge of activity coefficients, and the validity of the mathematical constructs used in obtaining DeltaG(tr) values. Regarding transfer from water to 1 M concentrations of the naturally occurring osmolytes, trimethylamine-N-oxide (TMAO), sarcosine, betaine, proline, glycerol, sorbitol, sucrose, trehalose, and urea, using cyclic glycylglycine, zwitterionic glycine peptides, and N-acetylglycine amide peptides as models for the peptide backbone of proteins, we set out to address these issues and obtain DeltaG(tr) values for the peptide backbone unit. We demonstrate experimental approaches that obviate the choice of concentration scale and demonstrate additivity in DeltaG(tr) of the peptide backbone unit for all solvent systems studied. Evidence is presented to show that the DeltaG(tr) values are independent of the chemical model studied, and experimental conditions are given to illustrate when the mathematical constructs are valid and when activity coefficients can be ignored. Resolution of the long-standing issues that have stymied development of the transfer model now make it possible to design transfer experiments that yield reliable and quantitative values for the interactions between osmolyte-containing solvents and native and unfolded protein.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
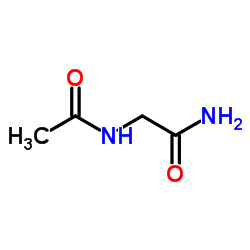 |
Nα-乙酰基甘氨酰胺
CAS:2620-63-5 |
C4H8N2O2 |
|
Characterization of the conformational probability of N-acet...
2005-06-23 [J. Phys. Chem. A 109(24) , 5289-302, (2005)] |
|
[The systems analysis of the effect of piracetam, glycine an...
1993-01-01 [Eksp. Klin. Farmakol. 56(4) , 55-7, (1993)] |
|
[Comparative influence of nootropic preparations on the emet...
1989-06-01 [Biull. Eksp. Biol. Med. 107(6) , 711-3, (1989)] |
|
Formation and stability of peptide enolates in aqueous solut...
2002-07-17 [J. Am. Chem. Soc. 124(28) , 8251-9, (2002)] |
|
Preserved acetazolamide reactivity in lacunar patients with ...
2012-05-01 [J. Cereb. Blood Flow Metab. 32(5) , 844-50, (2012)] |