Regio- and stereoselectivity of captodative olefins in 1,3-dipolar cycloadditions. A DFT/HSAB theory rationale for the observed regiochemistry of nitrones.
R Herrera, A Nagarajan, M A Morales, F Méndez, H A Jiménez-Vázquez, L G Zepeda, J Tamariz
文献索引:J. Org. Chem. 66 , 1252, (2001)
全文:HTML全文
摘要
Captodative olefins 1-acetylvinyl carboxylates proved to be highly regioselective dipolarophiles in 1,3-dipolar cycloadditon to propionitrile oxide, arylphenylnitrile imines, diazoalkanes, and nitrones to yield the corresponding 5-substituted heterocycles. The addition of the latter was also stereoselective, being slightly susceptible to steric demand of the carboxylate substituent in the olefin. All atempts to cleave the isoxazolidine N-O bond under reductive conditions failed, providing diverse products with side-group reduction. FMO theory was unsuccessful to explain the regioselectivity observed with nitrones, since the opposite orientation was predicted. The recently formulated DFT/HSAB theoretical model was able to rationalize this regioselectivity, identifying the nucleophilic and electrophilic atoms involved in the process via calculation of interaction energies, suggesting the specific direction of the electronic process at each of the reaction sites.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
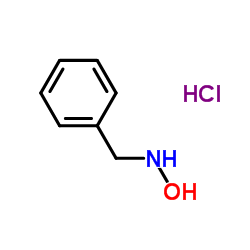 |
N-苄基羟胺盐酸盐
CAS:29601-98-7 |
C7H10ClNO |
|
NBHA reduces acrolein-induced changes in ARPE-19 cells: poss...
2011-04-01 [Curr. Eye Res. 36(4) , 370-8, (2011)] |
|
[1, 3]-Dipolar intramolecular nitrone olefin cycloaddition r...
[Tetrahedron Lett. 42(29) , 4925-28, (2001)] |
|
The Synthesis of Chiral Polyhydroxylated Pyrrolidines Using ...
[Synlett 10 , 1408-10, (2000)] |
|
A Large-Scale Low-Cost Preparation of N-Benzylhydroxy...
[Synthesis 18 , 3174-76, (2009)] |
|
J.E. Baldwin et al.
[Tetrahedron 42 , 3097, (1986)] |