Antiplasmodial structure-activity relationship of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide derivatives.
Adoración Marin, Lidia Moreira Lima, Beatriz Solano, Esther Vicente, Silvia Pérez Silanes, Séverine Maurel, Michel Sauvain, Ignacio Aldana, Antonio Monge, Eric Deharo
文献索引:Exp. Parasitol. 118(1) , 25-31, (2008)
全文:HTML全文
摘要
Derivatives of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide (4b-g, 5b-g, 6a-g) were synthesized and evaluated for their capacity to inhibit the growth of chloroquine-resistant Plasmodium falciparum FCB1 strain in culture. Compound 7-chloro-2-(2-furylcarbonyl)-3-trifluoromethyl-1,4-quinoxaline di-N-oxide (5g) was the most active being almost 5 times more active than chloroquine. It was also 50 times more active against P. falciparum than toxic toward MCF7 cells. Structural characteristics for a quinoxaline to be active were defined: bioisosteric modification of phenyl group by 2-thienyl or 2-furyl subunits, R2 position must be free or occupied by a methyl group and R1 position must be free or occupied by Cl, CH3, OCH3 or CF3.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
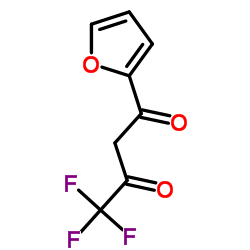 |
4,4,4-三氟-1-(2-呋喃基)-1,3-丁二酮
CAS:326-90-9 |
C8H5F3O3 |
|
4,7-Dichloro benzothien-2-yl sulfonylaminomethyl boronic aci...
2010-04-15 [Bioorg. Med. Chem. Lett. 20 , 2622-4, (2010)] |
|
Topography of the cristae membrane as elucidated by a new in...
1973-11-01 [Biochem. Biophys. Res. Commun. 55(1) , 169-73, (1973)] |
|
Induction of apoptosis by beta-diketones in human tumor cell...
2004-01-01 [Anticancer Res. 24(2B) , 711-7, (2004)] |
|
Formation of unusual glutamate conjugates of 1-[3-(aminometh...
2001-10-01 [Drug Metab. Dispos. 29(10) , 1296-306, (2001)] |
|
An efficient entry to perfluoroalkyl substituted azoles star...
[Tetrahedron 50(29) , 8827-8836 , (1994)] |