Photochemical transformation of acifluorfen under laboratory and natural conditions.
D Vialation, D Baglio, A Paya-Perez, C Richard
文献索引:Pest Manag. Sci. 57(4) , 372-9, (2001)
全文:HTML全文
摘要
Acifluorfen was irradiated in pure water at various excitation wavelengths and pH values. Numerous photoproducts were obtained which were identified by [1H]NMR and/or HPLC-MS/MS. The main reaction pathways were photo-decarboxylation, photo-cleavage of the ether bonding with formation of phenolic compounds, photo-dechlorination and photo-Claisen type rearrangement. Decarboxylation was observed in acidic and neutral media whereas cleavage of the ether bonding dominated in basic media. The photo-Claisen type rearrangement only occurred on excitation at short wavelengths. The quantum yield of photolysis was significantly lower at 313 nm (6.1 x 10(-5)) than at 254 nm (2.0 x 10(-3)). The photoreactivity of acifluorfen was then studied in conditions approaching environmental conditions. Acifluorfen was dissolved in pure water, in water containing humic substances or in a natural water, and exposed to solar light in June at Clermont-Ferrand (latitude 46 degrees N). In pure water, the half-life was estimated at 10 days and photo-decarboxylation accounted for 30% of the conversion. The presence of humic substances (10 mg litre-1) did not affect the rate of photo-transformation. However, the half-life of acifluorfen dissolved in the natural water was only 6.8 days.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
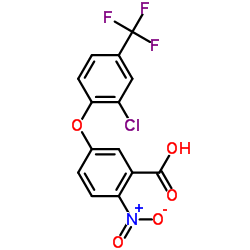 |
三氟羧草醚
CAS:50594-66-6 |
C14H7ClF3NO5 |
|
[Determination of biphenyl ether herbicides in water using H...
2010-01-01 [Sichuan Da Xue Xue Bao. Yi Xue Ban 41(1) , 148-52, (2010)] |
|
Increased expression of Fe-chelatase leads to increased meta...
2014-10-01 [Plant Mol. Biol. 86(3) , 271-87, (2014)] |
|
Photochemistry and photoinduced toxicity of acifluorfen, a d...
2002-01-01 [J. Environ. Qual. 31(1) , 268-74, (2002)] |
|
A continuous fluorimetric assay for protoporphyrinogen oxida...
2005-09-01 [Anal. Biochem. 344(1) , 115-21, (2005)] |
|
Impairment of the photosynthetic apparatus by oxidative stre...
2007-06-01 [Biochim. Biophys. Acta 1767(6) , 860-8, (2007)] |