Spectral studies on Co(II), Ni(II) and Cu(II) complexes with thiosemicarbazone (L1) and semicarbazone (L2) derived from 2-acetyl furan.
Sulekh Chandra, Anil Kumar
文献索引:Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 66(4-5) , 1347-51, (2007)
全文:HTML全文
摘要
Co(II), Ni(II) and Cu(II) complexes are synthesized with thiosemicarbazone (L1) and semicarbazone (L2) derived from 2-acetyl furan. These complexes are characterized by elemental analysis, molar conductance, magnetic susceptibility measurements, mass, IR, electronic and EPR spectral studies. The molar conductance measurements of the complexes in DMSO correspond to non-electrolytic nature except Ni(L)2(NO3)2, which is 1:2 electrolyte. All the complexes are of high-spin type. On the basis of spectral studies an octahedral geometry may be assigned for Co(II) and Ni(II) complexes except nitrato complexes of Ni(II) which is of tetrahedral geometry, whereas tetragonal geometry for Cu(II) complexes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
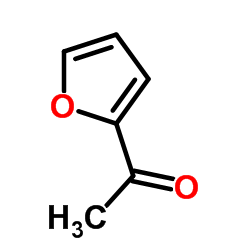 |
2-乙酰基呋喃
CAS:1192-62-7 |
C6H6O2 |
|
Quantification of furanic derivatives in fortified wines by ...
2015-02-13 [J. Chromatogr. A. 1381 , 54-63, (2015)] |
|
Dose-dependent increase in 2,5-hexanedione in the urine of w...
1991-01-01 [Int. Arch. Occup. Environ. Health 63(4) , 285-91, (1991)] |
|
2-Acetylfuran, a confounder in urinalysis for 2,5-hexanedion...
1991-01-01 [Int. Arch. Occup. Environ. Health 63(3) , 213-9, (1991)] |
|
[Acute hepatitis in subjects exposed to 2-acetylfuran and hy...
1983-01-01 [Med. Lav. 74(4) , 284-90, (1983)] |
|
Comparison of 2-acetylfuran formation between ribose and glu...
2008-12-24 [J. Agric. Food Chem. 56(24) , 11997-2001, (2008)] |