| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
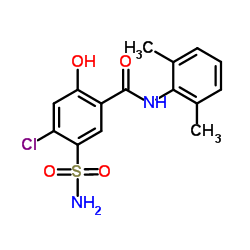 |
希伯胺
CAS:14293-44-8 |
|
 |
氨苯蝶啶
CAS:396-01-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
希伯胺
CAS:14293-44-8 |
|
 |
氨苯蝶啶
CAS:396-01-0 |