The other kind of biological NMR--studies of enzyme-substrate interactions.
G C Roberts
文献索引:Neurochem. Res. 21(9) , 1117-24, (1996)
全文:HTML全文
摘要
NMR spectroscopy has proved to be a valuable tool in the study of the interactions between enzymes and their substrates. The kinds of structural and dynamic information which can be obtained are illustrated by studies of three enzymes involved in drug metabolism. Cytochromes P450 play a crucial role in metabolism of a wide range of exogenous chemicals. NMR has been used to measure distances from the haem iron of the cytochrome to protons of the bound substrate, leading to detailed structural models for the enzyme-substrate complexes. The other two enzymes, chloramphenicol acetyltransferase and beta-lactamase, are responsible for bacterial resistance to specific antibiotics. In chloramphenicol acetyltransferase, NMR has been used to determine the conformation of coenzyme A bound to the enzyme, while in the case of beta-lactamase the pK of a specific lysine residue at the active site has been determined, providing valuable information on the catalytic mechanism.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
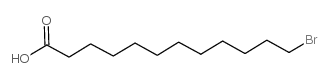 |
12-溴十二烷酸
CAS:73367-80-3 |
C12H23BrO2 |
|
12-Bromododecanoic acid binds inside the calyx of bovine bet...
1998-11-06 [FEBS Lett. 438(3) , 272-8, (1998)] |
|
Calibration of the channel that determines the omega-hydroxy...
2005-06-17 [J. Biol. Chem. 280(24) , 22697-705, (2005)] |
|
NMR studies of substrate binding to cytochrome P450 BM3: com...
1995-07-18 [Biochemistry 34(28) , 8982-8, (1995)] |
|
A simple synthesis of bromine-77-labeled alkyl bromides.
1982-05-01 [Int. J. Appl. Radiat. Isot. 33(5) , 391-2, (1982)] |