New herbicide models from benzoxazinones: aromatic ring functionalization effects.
Francisco A Macías, João M De Siqueira, Nuria Chinchilla, David Marín, Rosa M Varela, José M G Molinillo
文献索引:J. Agric. Food Chem. 54(26) , 9843-51, (2006)
全文:HTML全文
摘要
The utility of benzoxazinones and some of their synthetic derivatives in the search for new leads for herbicide model development has been widely discussed. As the benzoxazinone skeleton contains three different potential areas for functionalization (C-2, N-4, and aromatic protons H-5, H-6, H-7, and H-8), and the first two have already been optimized, the main objective of this work was the substitution of aromatic protons for different substituent types and the study of the effects of the prepared chemicals on selected standard target species (STS) and weeds. Thus, different combinations of aromatic substituents, including methoxy, methoxycarbonyl, fluorine, chlorine, and trifluoromethyl, were introduced at different positions. Phytotoxicity results were successfully correlated with steric and electronic molecular parameters, the resulting molecular volume (V) and dipole moment (mu) being the most influential ones. Halogenations at C-6 and fluorination at C-7 were the most successful modifications. Compounds 6-fluoro-(2H)-1,4-benzoxazin-3(4H)-one (6F-D-DIBOA), 7-fluoro-(2H)-1,4-benzoxazin-3(4H)-one (7F-D-DIBOA), and 6-chloro-(2H)-1,4-benzoxazin-3(4H)-one (6Cl-D-DIBOA) had the highest phytotoxic activities. The dose-response profiles on wheat and two of its most common weeds (Lolium rigidum Gaud. and Avena fatua L.) were compared by means of a proposed selectivity index, which displayed 7F-D-DIBOA as the most selective of the tested benzoxazinones.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
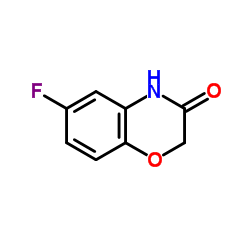 |
6-氟-2H-1,4-苯并噁唑-3(4H)-酮
CAS:398-63-0 |
C8H6FNO2 |
|
Peroxidase-catalyzed halide ion oxidation.
2000-01-01 [Redox Rep. 5(4) , 169-71, (2000)] |
|
The proximate coupling constant, 5 J (H, CH3), and the torsi...
[Can. J. Chem. 69(4) , 620-624, (1991)] |
|
Catalytic hydrogenation of sulfur-containing nitrobenzene ov...
[Appl. Catal. A Gen. 497 , 17-21, (2015)] |
|
Photoelectrochemical reduction of meta-halonitrobenzenes and...
[J. Chem. Soc., Perkin Trans. II 8 , 1673-1677, (1995)] |