Possible allostery and oligomerization of recombinant plastidial sn-glycerol-3-phosphate acyltransferase.
Xue Chen, Robin Miles, Crystal Snyder, Martin Truksa, Jian Zhang, Saleh Shah, Randall J Weselake
文献索引:Arch. Biochem. Biophys. 554 , 55-64, (2014)
全文:HTML全文
摘要
Plastidial acyl-acyl carrier protein:sn-glycerol-3-phosphate acyltransferase (GPAT; EC 2.3.1.15) catalyzes the acyl-acyl carrier protein-dependent sn-1 acylation of sn-glycerol 3-phosphate (G3P) to produce lysophosphatic acid. Functional recombinant Erysimum asperum GPAT (EaGPAT), devoid of transit peptide, was produced in yeast. Analysis of the dependence of EaGPAT activity on increasing G3P concentration resulted in a hyperbolic response. EaGPAT exhibited a preference for 18-carbon unsaturated acyl-CoAs. Assays with concentrations of oleoyl-CoA up to 90μM revealed an exponential response to increasing concentrations of acyl donor, and the introduction of increasing concentrations of unlabeled linoleoyl-CoA into the standard reaction mixture resulted in increased incorporation of radiolabeled oleoyl moieties into lysophosphatidic acid. Collectively, the kinetic results suggest that acyl-CoA may act as both substrate and allosteric effector. EaGPAT was also shown to oligomerize to form higher molecular mass multimers, with the monomer and trimer being the predominant forms of the enzyme. Since most allosteric enzyme exhibit quaternary structure, the self-associating properties of EaGPAT are consistent with those of an allosteric enzyme. These results could have important regulatory implications when plastidial GPAT is introduced into a cytoplasmic environment where acyl-CoA is the acyl donor supporting cytoplasmic glycerolipid assembly.Copyright © 2014 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
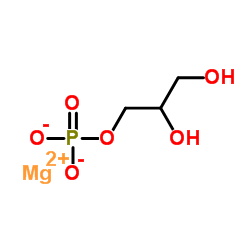 |
甘油磷酸镁
CAS:927-20-8 |
C3H7MgO6P |
|
One-pot four-enzyme synthesis of ketoses with fructose 1,6-b...
2012-08-01 [Carbohydr. Res. 357 , 143-6, (2012)] |
|
The Pochonia chlamydosporia serine protease gene vcp1 is sub...
2012-01-01 [PLoS ONE 7(4) , e35657, (2012)] |
|
Crystal structures of triosephosphate isomerase from methici...
2012-12-01 [Biochimie 94(12) , 2532-44, (2012)] |
|
The lipid A from Vibrio fischeri lipopolysaccharide: a uniqu...
2011-06-17 [J. Biol. Chem. 286(24) , 21203-19, (2011)] |
|
Thyroid hormone contributes to the hypolipidemic effect of p...
2011-10-01 [J. Endocrinol. 211(1) , 65-72, (2011)] |