Photoinactivation of peptide transport in Saccharomyces cerevisiae.
J M Becker, K P Dunsmore, A S Steinfeld, F Naider
文献索引:Biochemistry 21(23) , 5967-71, (1982)
全文:HTML全文
摘要
Oligopeptides and dipeptides are transported into Saccharomyces cerevisiae by a carrier-mediated system. In the dark, leucyl-p-nitroanilide (Leu-p-NA) and leucyl-leucyl-4-azido-2-nitrophenylalanine [Leu-Leu-Phe-(4N3,2NO2)] are competitive inhibitors of peptide transport by S. cerevisiae cells. The photolysis of yeast cells in the presence of Leu-p-NA or Leu-Leu-Phe(4N3,2NO2) at 350 nm results in an irreversible inactivation of peptide transport. Protection against this inactivation is afforded by an excess of trimethionine, a transported peptide. Photolysis with Leu-p-NA or Leu-Leu-Phe(4N3,2NO2) does not affect amino acid or sugar transport, and cell viability is maintained throughout the irradiation procedure. A 5-min irradiation of S. cerevisiae with 2.4 microM Leu-p-NA or 15 microM Leu-Leu-Phe(4N3,2NO2) causes 50% inhibition of trimethionine uptake. p-Nitroaniline, a possible hydrolysis product generated from Leu-p-NA by cellular peptidase activity, has no effect on peptide transport. An exogenous energy source is not required for photoinactivation. The results suggest that a component(s) of the peptide transport system of S. cerevisiae is irreversibly modified by photolysis with Leu-p-NA or Leu-Leu-Phe-(4N3,2NO2) and provide the first example of the use of amino acid p-nitroanilides as photoaffinity labels.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
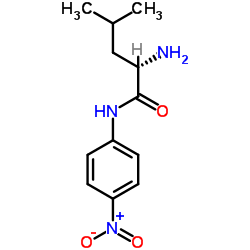 |
L-亮氨酸-4-硝基苯胺
CAS:4178-93-2 |
C12H17N3O3 |
|
Screening the Medicines for Malaria Venture "Malaria Box" ag...
2015-01-01 [PLoS ONE 10(2) , e0115859, (2015)] |
|
Beta-phenyl cysteine: a leucine aminopeptidase inhibitor.
1984-06-01 [Pharmacol. Res. Commun. 16(6) , 533-8, (1984)] |
|
Exploration of structural and physicochemical requirements a...
2013-02-01 [Mol. Divers. 17(1) , 123-37, (2013)] |
|
Covalent immobilisation of protease and laccase substrates o...
2010-08-01 [Chemosphere 80(8) , 922-8, (2010)] |
|
The leucyl aminopeptidase from Helicobacter pylori is an all...
2005-06-01 [Microbiology 151(Pt 6) , 2017-23, (2005)] |