Functional glass surface displaying a glutamyl donor substrate for transglutaminase-mediated protein immobilization.
Kyunga Sung, Noriho Kamiya, Noriyuki Kawata, Shinji Kamiya, Masahiro Goto
文献索引:Biotechnol. J. 5 , 456-462, (2010)
全文:HTML全文
摘要
A chemically modified glass surface displaying a glutamyl donor substrate peptide (Z-QG) was developed for microbial transglutaminase (MTG)-mediated immobilization of recombinant proteins tagged with an MTG-reactive lysine-containing substrate peptide (K-tag). To evaluate the surface modification conditions affecting the enzymatic protein immobilization, we employed an amino-modified 96-well glass plate as a base and prepared three types of glass surfaces displaying Z-QG. Validation of the Z-QG modified glass surfaces with recombinant enhanced green fluorescent proteins revealed that the insertion of a di(ethylene glycol) linker between the terminal Z-QG moiety and the base not only enhances enzymatic protein immobilization efficiency but also decreases nonselective protein adsorption. A bacterial alkaline phosphatase fused with a K-tag at the N terminus was also successfully immobilized to the designed glass surface, suggesting that the chemically modified glass surface displaying a spatially controlled glutamyl donor substrate is a potential platform for MTG-mediated fabrication of protein-based solid biomaterials.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
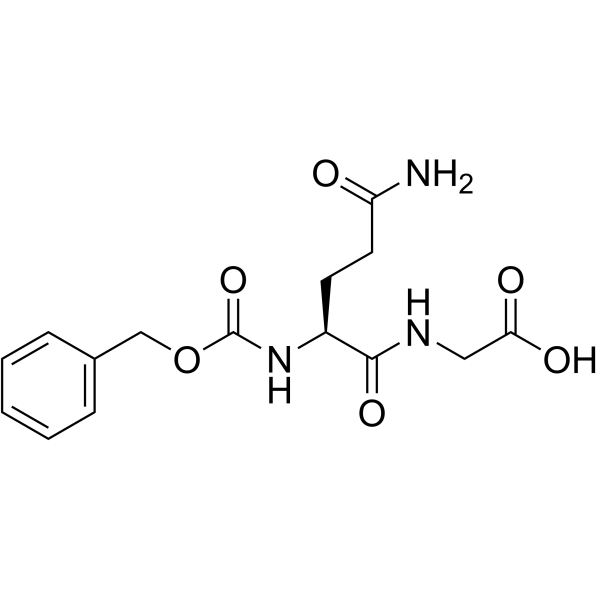 |
Z-谷氨酰甘氨酸
CAS:6610-42-0 |
C15H19N3O6 |
|
A direct continuous spectrophotometric assay for transglutam...
2000-10-01 [Anal. Biochem. 285 , 16-20, (2000)] |
|
Chemoenzymatic synthesis of neoglycopeptides: application to...
2001-05-04 [J. Org. Chem. 66 , 2948-56, (2001)] |
|
Kinetic analysis of the action of tissue transglutaminase on...
2003-08-12 [Biochemistry 42 , 9466-9481, (2003)] |
|
Transglutaminase-mediated synthesis of a DNA-(enzyme)n probe...
2011-05-02 [Chemistry 17 , 5387-5392, (2011)] |
|
Enzymatically catalyzed HES conjugation using microbial tran...
2009-11-01 [J. Pharm. Sci. 98 , 4420-4428, (2009)] |