| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
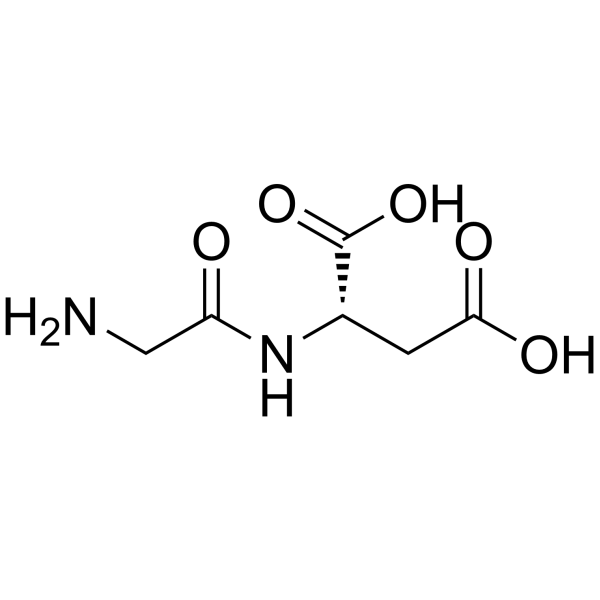 |
甘氨酰-L-天冬氨酸
CAS:4685-12-5 |
|
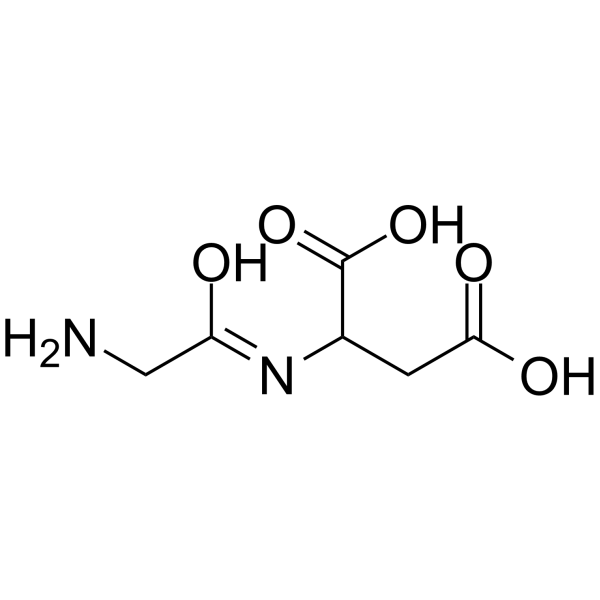 |
甘氨酰-DL-天冬氨酸
CAS:79731-35-4 |