Core-clickable PEG-branch-azide bivalent-bottle-brush polymers by ROMP: grafting-through and clicking-to.
Jeremiah A Johnson, Ying Y Lu, Alan O Burts, Yeon-Hee Lim, M G Finn, Jeffrey T Koberstein, Nicholas J Turro, David A Tirrell, Robert H Grubbs
文献索引:J. Am. Chem. Soc. 133 , 559-566, (2011)
全文:HTML全文
摘要
The combination of highly efficient polymerizations with modular "click" coupling reactions has enabled the synthesis of a wide variety of novel nanoscopic structures. Here we demonstrate the facile synthesis of a new class of clickable, branched nanostructures, polyethylene glycol (PEG)-branch-azide bivalent-brush polymers, facilitated by "graft-through" ring-opening metathesis polymerization of a branched norbornene-PEG-chloride macromonomer followed by halide-azide exchange. The resulting bivalent-brush polymers possess azide groups at the core near a polynorbornene backbone with PEG chains extended into solution; the structure resembles a unimolecular micelle. We demonstrate copper-catalyzed azide-alkyne cycloaddition (CuAAC) "click-to" coupling of a photocleavable doxorubicin (DOX)-alkyne derivative to the azide core. The CuAAC coupling was quantitative across a wide range of nanoscopic sizes (∼6-∼50 nm); UV photolysis of the resulting DOX-loaded materials yielded free DOX that was therapeutically effective against human cancer cells.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
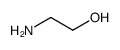 |
O-(2-氨基乙基)聚乙二醇3,000
CAS:32130-27-1 |
C2H7NO |
|
Particles without a box: brush-first synthesis of photodegra...
2013-01-01 [J. Vis. Exp. (80) , doi:10.3791/50874, (2013)] |
|
Drug-loaded, bivalent-bottle-brush polymers by graft-through...
2012-01-01 [Macromolecules 43 , 10326-10335, (2010)] |
|
Redox-responsive branched-bottlebrush polymers for in vivo M...
2014-01-01 [Nat. Commun. 5 , 5460, (2014)] |
|
Synthesis of Acid-Labile PEG and PEG-Doxorubicin-Conjugate N...
2014-09-01 [ACS Macro Lett. 3(9) , 854-857, (2014)] |
|
A convergent synthetic platform for single-nanoparticle comb...
2014-04-23 [J. Am. Chem. Soc. 136(16) , 5896-9, (2014)] |