Residues of oxytetracycline and its 4'-epimer in edible tissues from turkeys.
Francesca Capolongo, Annalisa Santi, Luciana Tomasi, Paola Anfossi, Mauro Missagia, Clara Montesissa
文献索引:J. AOAC Int. 85(1) , 8-14, (2002)
全文:HTML全文
摘要
Residues of oxytetracycline (OTC) in edible tissues (muscle, liver, and kidney) of 18 turkeys were determined after continuous administration of the drug for 3 days in drinking water at the maximum recommended concentration of 400 mg/L. The European Union (EU) maximum residue limits (MRLs) set for OTC are 100 microg/kg in muscle tissues, 300 microg/kg in liver, and 600 microg/kg in kidney, as the sum of the parent compound and its derivative 4'-epi-oxytetracycline (4-epi-OTC). Cleanup of tissue samples was performed by metal chelate affinity chromatography (MCAC), but the original technique was miniaturized by the adoption of a mini solid-phase extraction column, allowing reduction of solvents, time, and hazardous waste. OTC and its 4'-epimer were quantitated by an isocratic liquid chromatography elution with UV detection. After 1 day of withdrawal, OTC plus 4-epi-OTC residues were greater than MRL values in muscle and liver; 3 days after the end of treatment, all tissue residues were far lower than the MRL values. At the first day after the end of treatment, 4-epi-OTC was detected at very low concentrations only in muscle, in liver after 1 and 3 days of withdrawal, and in kidney at all sampling times. The withdrawal time was calculated according to EU recommendations and was set at 5 days.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
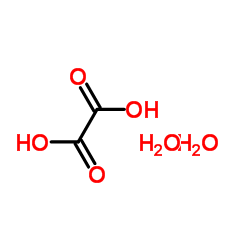 |
草酸
CAS:6153-56-6 |
C2H6O6 |
|
Lefetamine, a controlled drug and pharmaceutical lead of new...
2015-02-01 [Anal. Bioanal. Chem 407(6) , 1545-57, (2015)] |
|
An Assessment of Engineered Calcium Oxalate Crystal Formatio...
2015-01-01 [PLoS ONE 10 , e0141982, (2015)] |
|
Thermodynamics for complex formation between palladium(ii) a...
2014-08-28 [Dalton Trans. 43(32) , 12243-50, (2014)] |
|
An olive-shaped SnO2 nanocrystal-based low concentration H2S...
2015-08-28 [Phys. Chem. Chem. Phys. 17 , 20537-42, (2015)] |
|
13C chemical shielding in oxalic acid, oxalic aci...
[J. Chem. Phys. 63(3) , 1267-71, (1975)] |