Characterization of an aminopeptidase and a proline iminopeptidase from cabbage leaves.
Margarita Marinova, Alexander Dolashki, Florian Altenberend, Stefan Stevanovic, Wolfgang Voelter, Bozhidar Tchorbanov
文献索引:Z. Naturforsch., C, J. Biosci. 63 , 105-112, (2008)
全文:HTML全文
摘要
Aminopeptidase, preferring phenylalanine-p-nitroanilide as substrate, and proline iminopeptidase, highly-specific for proline-p-nitroanilide, were isolated from cabbage leaves (Brassica oleraceae var. capitata). As pH optima, 7.2-7.5 for aminopeptidase activity and 8.0-8.5 for proline iminopeptidase were determined. Both peptidases were strongly inhibited by p-chloromercuribenzoic acid, heavy metal ions and urea. The molecular weights were determined by gel filtration to be 56 and 204 kDa, respectively. The iminopeptidase was decomposed during SDS electrophoresis to four subunits of 50 kDa. Minor impurities of myrosinase-associated protein (approximately 70 kDa) were found in both preparations. Preliminary data of their amino acid sequences showed similarities to those of aminopeptidases N (family M1) and proline iminopeptidases (family S33).
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
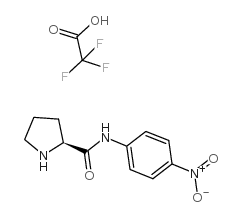 |
L-脯氨酸对硝基苯胺三氟乙酸盐
CAS:108321-19-3 |
C13H14F3N3O5 |
|
Generation of food-grade recombinant Lactobacillus casei del...
2014-08-01 [Appl. Microbiol. Biotechnol. 98(15) , 6689-700, (2014)] |
|
Synthesis and biological evaluation of nigranoic acid esters...
2015-01-01 [Nat. Prod. Res. 29 , 1650-6, (2015)] |
|
Characterization of a multimeric, eukaryotic prolyl aminopep...
2009-11-01 [Microbiology 155 , 3673-3682, (2009)] |
|
Collagen production in cardiac fibroblasts during inhibition...
2005-09-01 [J. Renin Angiotensin Aldosterone Syst. 6 , 69-77, (2005)] |
|
Purification and characterization of a novel prolyl aminopep...
2004-06-01 [Biosci. Biotechnol. Biochem. 68 , 1395-7, (2004)] |