A pharmacokinetic and endoscopic comparison of an oral and an experimental buccal piroxicam formulation.
B A Mueller, D K Rex, N Figueroa, P Greene, D C Brater
文献索引:Pharmacotherapy 12(2) , 93-7, (1992)
全文:HTML全文
摘要
We compared the endoscopic effects and pharmacokinetic profiles of an experimental buccal formulation of piroxicam to oral capsules in an attempt to determine whether nonsteroidal antiinflammatory drug-induced gastropathy is due to a local or systemic effect. Ten healthy subjects received 20 mg piroxicam daily in a double-blind, randomized, crossover, placebo-controlled study. Upper endoscopies were performed at the baseline and at the end of each 2-week dosing arm of the study. Pharmacokinetic data obtained included serum and gastric piroxicam concentrations and serum 5'-hydroxypiroxicam metabolite concentrations after the first dose and 2 weeks of dosing. No differences in endoscopy scores or patient symptom scores were noted between the two dosage forms after 2 weeks of dosing. Pharmacokinetic data of piroxicam and the metabolite revealed that the buccal formulation may not have been absorbed exclusively from the buccal mucosa.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
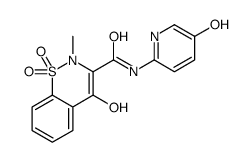 |
5-羟基吡罗昔康
CAS:77459-78-0 |
C15H13N3O5S |
|
[The binding of oxicam derivatives to human serum albumin].
1988-08-01 [Arzneimittelforschung 38(8) , 1089-92, (1988)] |
|
High-performance liquid chromatographic analysis of piroxica...
[J. Chromatogr. A. 382 , 382-8, (1986)] |
|
Determination of piroxicam and its major metabolites in the ...
[J. Chromatogr. A. 576(1) , 121-8, (1992)] |
|
Extractionless high-performance liquid chromatographic metho...
1995-11-03 [J. Chromatogr. B, Biomed. Appl. 673(1) , 142-6, (1995)] |
|
Simultaneous determination of piroxicam and its major metabo...
1998-08-01 [Analyst 123(8) , 1749-52, (1998)] |