Incidence and clinical course of lercanidipine-associated cloudy effluent in continuous ambulatory peritoneal dialysis.
P-J Hsiao, H-W Lin, C-C Sung, C-W Wang, P Chu, S-H Lin
文献索引:Clin. Nephrol. 74(3) , 217-22, (2010)
全文:HTML全文
摘要
Lercanidipine, a novel dihydropyridine calcium channel antagonist, has been reported to cause sterile cloudy effluent in patients on continuous ambulatory peritoneal dialysis (CAPD). The purpose of the study was to evaluate the incidence and clinical course of cloudy effluent associated with lercanidipine in uremic patients on CAPD.We designed a consecutive observation study in 40 non-diabetic uremic patients on CAPD treated with lercanidipine 5 mg daily. Lercanidipine-induced cloudy effluent was defined as acellular and culture-negative effluent associated with the use of this drug and exclusion of other causative factors. Time to develop cloudy effluent, dwell effluent amount and the associated symptoms were recorded. Baseline peritoneal membrane characteristics, net ultrafiltration per session and routine biochemistry in serum and dialysate were compared between patients with and without the development of cloudy effluent.9 patients (22.5%) developed cloudy effluent within 2 days of lercanidipine initiation. The triglyceride concentration in cloudy effluent was greater than 10 mg/dl (19.3 ± 6.3 mg/dl). There was a significant increase in dwell effluent amount (93.3 ± 64 ml/exchange, p < 0.05). Clinical symptoms as abdominal cramping or fullness were observed in 3 patients. All cloudy effluent disappeared after ceasing lercanidipine but recurred after resumption of lercanidipine. Baseline dialysate to plasma (D/P) creatinine ratio (0.7 ± 0.1 vs. 0.51 ± 0.1; p = 0.07) tended to be higher and dialysate total protein (93.4 ± 33 vs. 61.5 ± 24 mg/dl; p < 0.05) were significantly higher in patients with than without the development of cloudy effluent.The incidence of lercanidipine-associated cloudy effluent is relatively higher with transient benign clinical symptoms. Patients with lercanidipine associated cloudy effluent tend to have a higher membrane transport with an increased effluent amount.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
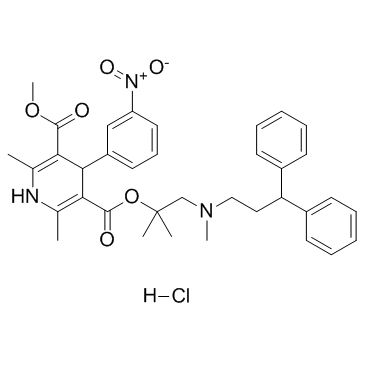 |
盐酸乐卡地平
CAS:132866-11-6 |
C36H42ClN3O6 |
|
Differences in lercanidipine systemic exposure when administ...
2012-07-01 [Eur. J. Clin. Pharmacol. 68(7) , 1043-7, (2012)] |
|
Rationale for the use of a fixed-dose combination in the man...
2010-01-01 [Clin. Drug Investig. 30(12) , 843-54, (2010)] |
|
Lercanidipine rescues hippocampus pyramidal neurons from mil...
2011-05-01 [Cell. Mol. Neurobiol. 31(4) , 561-7, (2011)] |
|
Polymorphism of the methylenetetrahydrofolate reductase gene...
2012-06-01 [Gene 500(2) , 207-10, (2012)] |
|
Efficacy and tolerability of the fixed lercanidipine-enalapr...
2010-01-01 [Arzneimittelforschung 60(3) , 124-30, (2010)] |