Effects of external electromagnetic fields on the conformational sampling of a short alanine peptide.
Gleb Y Solomentsev, Niall J English, Damian A Mooney
文献索引:J. Comput. Chem. 33(9) , 917-23, (2012)
全文:HTML全文
摘要
Non-equilibrium molecular dynamics simulations of a solvated 21-residue polyalanine (A21) peptide, featuring a high propensity for helix formation, have been performed at 300 K and 1 bar in the presence of external electromagnetic (e/m) fields in the microwave region (2.45 GHz) and an r.m.s. electric field intensity range of 0.01-0.05 V/Å. To investigate how the field presence affects transitions between the conformational states of a protein, we report 16 independent 40 ns-trajectories of A21 starting from both extended and fully folded states. We observe folding-behavior of the peptide consistent with prior simulation and experimental studies. The peptide displays a natural tendency to form stable elements of secondary structure which are stabilized by tertiary interactions with proximate regions of the peptide. Consistent with our earlier work, the presence of external e/m fields disrupts this behavior, involving a mechanism of localized dipolar alignment which serves to enhance intra-protein perturbations in hydrogen bonds (English, et al., J. Chem. Phys. 2010, 133, 091105), leading to more frequent transitions between shorter-lifetime states.Copyright © 2012 Wiley Periodicals, Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
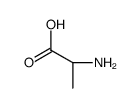 |
聚-DL-丙氨酸
CAS:25281-63-4 |
C3H7NO2 |
|
Enzyme-degradable self-assembled hydrogels from polyalanine-...
2013-02-12 [Macromol. Rapid Commun. 34(3) , 257-62, (2013)] |
|
Exploring the energy landscapes of protein folding simulatio...
2012-02-22 [Biophys. J. 102(4) , 878-86, (2012)] |
|
Comparison of some dispersion-corrected and traditional func...
2012-07-28 [J. Chem. Phys. 137(4) , 044109, (2012)] |
|
Simulated IR, isotropic and anisotropic Raman, and vibration...
2012-04-12 [J. Phys. Chem. B 116(14) , 4141-53, (2012)] |
|
Depletion of cognate charged transfer RNA causes translation...
2013-01-31 [Cell Rep. 3(1) , 148-59, (2013)] |