Involvement of lysine residues in the binding of ovine prolactin and human growth hormone to lactogenic receptors.
P de la Llosa, N Chêne, J Martal
文献索引:FEBS Lett. 191(2) , 211-5, (1985)
全文:HTML全文
摘要
The lactogenic activity (L.A.) of oPRL and hGH derivatives obtained by chemical modifications of lysine residues was studied by radioreceptor assay. Control treatment with borohydride had a slight effect on the L.A. of hGH but drastically reduced the oPRL activity; this latter was preserved in the presence of iodoacetamide. Methylation, ethylation, guanidination and acetimidination affected the L.A. of both hormones as a function of the degree of modification. The structure-binding relationships to the lactogenic receptors are discussed, suggesting that the lysine or arginine residues in homologous positions 42, 51, 73, 128, 146 of oPRL and 47, 50, 73, 128, 147 of hGH might be particularly involved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
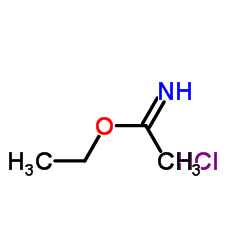 |
乙基乙酰亚胺盐酸盐
CAS:2208-07-3 |
C4H10ClNO |
|
Involvement of lysine residues in the binding of ovine chori...
1984-01-30 [FEBS Lett. 166(2) , 352-6, (1984)] |
|
Rapid removal of acetimidoyl groups from proteins and peptid...
1981-11-01 [Biochem. J. 199(2) , 335-40, (1981)] |
|
Studies on the structure of the ligand-binding site of the b...
1992-07-22 [Biochem. Pharmacol. 44(2) , 325-34, (1992)] |
|
An alkyl imidate labeling study of the organization of phosp...
1985-01-10 [J. Biol. Chem. 260(1) , 663-71, (1985)] |
|
The molecular basis of the low hemolytic activity of C4 mole...
1989-09-01 [Eur. J. Immunol. 19(9) , 1613-8, (1989)] |