Experimental and computational thermochemical study of barbituric acids: structure-energy relationship in 1,3-dimethylbarbituric acid.
María Victoria Roux, Rafael Notario, Concepción Foces-Foces, Manuel Temprado, Francisco Ros, Vladimir N Emel'yanenko, Sergey P Verevkin
文献索引:J. Phys. Chem. A 115(14) , 3167-73, (2011)
全文:HTML全文
摘要
This paper reports an experimental and computational thermochemical study on 1,3-dimethylbarbituric acid. The value of the standard (p° = 0.1 MPa) molar enthalpy of formation in the gas phase at T = 298.15 K has been determined. The energy of combustion was measured by static bomb combustion calorimetry, and from the result obtained, the standard molar enthalpy of formation in the crystalline state at T = 298.15 K was calculated as -639.6 ± 1.9 kJ·mol(-1). The enthalpy of sublimation was determined using a transference (transpiration) method in a saturated N(2) stream and a value of the enthalpy of sublimation at T = 298.15 K was derived as 92.3 ± 0.6 kJ·mol(-1). From these results a value of -547.3 ± 2.0 kJ·mol(-1) for the gas-phase enthalpy of formation at T = 298.15 K was determined. Theoretical calculations at the G3 and G4 levels were performed, and a study on molecular and electronic structure of the compound has been carried out. Calculated enthalpies of formation are in very good agreement with the experimental value.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
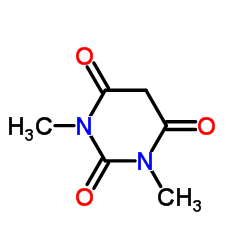 |
1,3-二甲基巴比妥酸
CAS:769-42-6 |
C6H8N2O3 |
|
Organocatalytic Michael-Knoevenagel-hetero-Diels-Alder react...
2012-01-20 [Org. Lett. 14(2) , 448-51, (2012)] |
|
Self-assembled Pd6 open cage with triimidazole walls and the...
2012-09-24 [Chemistry 18(39) , 12322-9, (2012)] |
|
Multicomponent self-sorting of a Pd7 molecular boat and its ...
2013-05-14 [Chem. Commun. (Camb.) 49(39) , 4307-9, (2013)] |
|
Synthesis of 5-aryl-1,3-dimethyl-6-(alkyl- or aryl-amino) fu...
2011-02-01 [Mol. Divers. 15(1) , 227-31, (2011)] |
|
Sequential one-pot bimetallic Ir(III)/Pd(0) catalysed mono-/...
2006-12-28 [Chem. Commun. (Camb.) (48) , 5000-2, (2006)] |