| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
碘化物阴离子标准液
CAS:12027-06-4 |
|
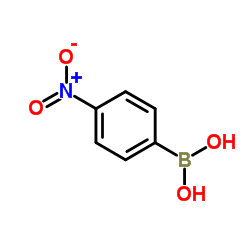 |
4-硝基苯硼酸
CAS:24067-17-2 |
|
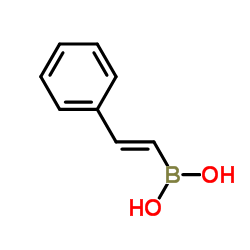 |
反式-BETA-苯乙烯硼酸
CAS:6783-05-7 |
|
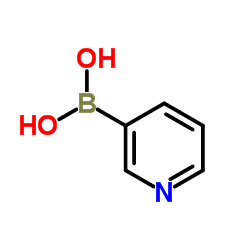 |
吡啶-3-硼酸
CAS:1692-25-7 |