Organic Letters
2007-07-19
Enantioselective total synthesis of cyathin A3.
Dale E Ward, Jianheng Shen
文献索引:Org. Lett. 9(15) , 2843-6, (2007)
全文:HTML全文
摘要
The total synthesis of (-)-cyathin A3 is described. The key step involves an unusual enantioselective Diels-Alder reaction of 2,5-dimethyl-1,4-benzoquinone with 2,4-bis(trimethylsilyloxy)-1,3-pentadiene, using Mikami's catalyst [(R)-BINOL + Cl2Ti(OiPr)2 + 4 A mol sieves] modified by addition of Mg and SiO2. Because cyathin A3 is easily transformed into allocyathin B3, cyathin B3, cyathin C3, and neoallocyathin A4, this route also constitutes formal syntheses of these natural products.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
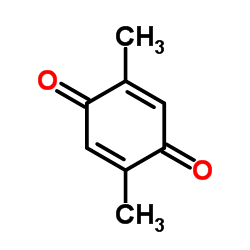 |
2,5-二甲基-1,4-苯醌
CAS:137-18-8 |
C8H8O2 |
相关文献:
更多...
|
Chemical defense of an opilionid (Acanthopachylus aculeatus)...
2004-03-01 [J. Exp. Biol. 207(Pt 8) , 1313-21, (2004)] |
|
Inhibition of jack bean urease by 1,4-benzoquinone and 2,5-d...
2002-08-01 [J. Enzyme Inhib. Med. Chem. 17(4) , 247-53, (2002)] |
|
Quinone-induced inhibition of urease: elucidation of its mec...
2007-06-01 [Bioorg. Chem. 35(3) , 233-42, (2007)] |
|
Chlorophyll a fluorescence transient as an indicator of acti...
1990-02-02 [Biochim. Biophys. Acta 1015(2) , 180-8, (1990)] |
|
An antiproliferative bis-prenylated quinone from the New Zea...
2007-12-01 [J. Nat. Prod. 70 , 2042-4, (2007)] |