The metal function in the reactions of bovine serum amine oxidase with substrates and hydrazine inhibitors.
G De Matteis, E Agostinelli, B Mondovì, L Morpurgo
文献索引:J. Biol. Inorg. Chem. 4(3) , 348-53, (1999)
全文:HTML全文
摘要
Bovine serum amine oxidase (BSAO) reacts with 2-hydrazinopyridine, which binds the organic co-factor 2,4,5-trihydroxyphenylalanine quinone, forming a band at 435 nm. The band shifts to 526 nm around 60 degrees C, to 415 nm upon denaturation, but only shifts to 429 nm upon Cu2+ depletion. Its wavelength and intensity suggest that the adduct has the azo conformation, whilst the same adduct of crystalline Escherichia coli amine oxidase (ECAO) shows the hydrazone conformation in the X-ray structure. The steady state kinetics of aminomethyl- and aminoethylpyridines confirm that the formation of the product Schiff base, analogous to the azo form of the 2-hydrazinopyridine adduct, is not hindered in solution. The structural stability of the adduct in the absence of Cu2+ is taken to imply hydrogen bonding of the pyridyl nitrogen to a conserved aspartate, as in the ECAO adduct. Thus the ECAO adduct provides a good model for a transient intermediate leading to formation of the BSAO azo adduct. On the basis of this model and of the catalytic competence of Co(2+)-substituted BSAO, confirmed by the present data, a catalytic reaction scheme is proposed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
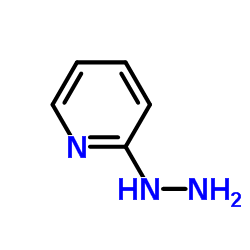 |
2-肼吡啶
CAS:4930-98-7 |
C5H7N3 |
|
Steroid hormones in prediction of normal pressure hydrocepha...
2015-08-01 [J. Steroid Biochem. Mol. Biol. 152 , 124-32, (2015)] |
|
Simultaneous determination of 17alpha-hydroxypregnenolone an...
2008-09-10 [J. Pharm. Biomed. Anal. 48(1) , 177-82, (2008)] |
|
Liquid chromatography-tandem mass spectrometric method for d...
2008-05-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 867(1) , 49-56, (2008)] |
|
Quantification of 2-hydrazinopyridine derivatized steroid ho...
2011-03-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(9-10) , 591-8, (2011)] |
|
Salivary chenodeoxycholic acid and its glycine-conjugate: th...
2010-04-01 [Steroids 75(4-5) , 338-45, (2010)] |