| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
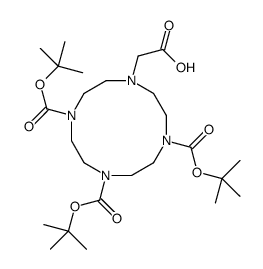 |
1,4,7-三-Boc-10-(羧甲基)-1,4,7,10-四氮杂环十二烷
CAS:247193-74-4 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
1,4,7-三-Boc-10-(羧甲基)-1,4,7,10-四氮杂环十二烷
CAS:247193-74-4 |