The difluorotoluene debate--a decade later.
Eric T Kool, Herman O Sintim
文献索引:Chem. Commun. (Camb.) (35) , 3665-75, (2006)
全文:HTML全文
摘要
2,4-Difluorotoluene is unusual among hydrofluorocarbons because it is shaped like the DNA base thymine. It was first synthesised as a nucleotide analogue and incorporated into DNA a decade ago. Although it is a nonpolar molecule, it was found to be replicated by DNA polymerase enzymes as if it were thymine. We concluded that replication of DNA base pairs can occur without Watson-Crick hydrogen bonds, and hypothesised that steric effects, rather than these hydrogen bonds, were the main arbiters of DNA replication fidelity. A debate was initiated then, with claims by some that the molecule is polar and forms hydrogen bonds with adenine, thus supporting the hydrogen bonding theory of DNA replication. Here we discuss the evolution of this debate, and reflect on the relevant data that have since come from hundreds of papers and dozens of laboratories. Although discussion on this topic continues, the steric hypothesis for DNA replication is now widely accepted among biochemists, and the changing paradigm has been reflected in textbooks.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
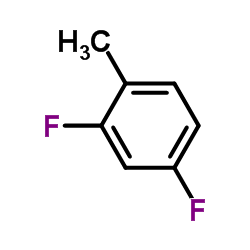 |
2,4-二氟甲苯
CAS:452-76-6 |
C7H6F2 |
|
A thymine isostere in the templating position disrupts assem...
2005-11-22 [Biochemistry 44(46) , 15230-7, (2005)] |
|
Importance of terminal base pair hydrogen-bonding in 3'-end ...
2000-03-14 [Biochemistry 39(10) , 2626-32, (2000)] |
|
Nonpolar nucleobase analogs illuminate requirements for site...
2006-11-24 [J. Biol. Chem. 281(47) , 35914-21, (2006)] |
|
Molecular moment similarity between several nucleoside analo...
1999-06-01 [J. Biomol. Struct. Dyn. 16(6) , 1169-75, (1999)] |
|
Quantum-chemical ab initio study on the adenine-difluorotolu...
1997-12-01 [J. Biomol. Struct. Dyn. 15(3) , 619-24, (1997)] |