Flexible protocol for the chemo- and regioselective building of pyrroles and pyrazoles by reactions of Danishefsky's dienes with 1,2-diaza-1,3-butadienes.
Orazio A Attanasi, Gianfranco Favi, Paolino Filippone, Gianluca Giorgi, Fabio Mantellini, Giada Moscatelli, Domenico Spinelli
文献索引:Org. Lett. 10(10) , 1983-6, (2008)
全文:HTML全文
摘要
The versatility of the Mukaiyama-Michael-type addition/heterocyclization of Danishefsky's diene with 1,2-diaza-1,3-butadienes was applied to the synthesis of both 4 H-1-aminopyrroles and 4,5 H-pyrazoles. Thus, the same reagents furnished different types of highly functionalized azaheterocycles essentially depending on their structure: as a matter of fact, R1 = COOR or CONR 2 differently affects the acidity of the proton at the adjacent carbon. An unexpected formation of 5 H-1-aminopyrroles from the reactions carried out in water was also observed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
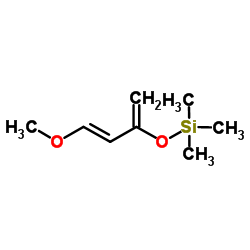 |
反-1-甲氧基-3-(三甲基硅氧基)-1,3-丁二烯
CAS:54125-02-9 |
C8H16O2Si |
|
Highly selective Diels-Alder reactions of directly connected...
2007-01-24 [J. Am. Chem. Soc. 129 , 645, (2007)] |
|
Cross-Diels-Alder reactions of 6-oxo-1-sulfonyl-1,6-dihydrop...
2007-03-30 [J. Org. Chem. 72 , 2364, (2007)] |
|
Sulfogriseofulvin derivatives. synthesis by [4 + 2]cycloaddi...
1996-07-01 [Arch. Pharm. (Weinheim) 329(7) , 361-70, (1996)] |
|
[Tetrahedron 49 , 1749, (1993)] |
|
[J. Org. Chem. 57 , 3605, (1992)] |