Journal of Pharmaceutical Sciences
1983-02-01
Kinetics and thermodynamics of interfacial transfer.
R Fleming, R H Guy, J Hadgraft
文献索引:J. Pharm. Sci. 72(2) , 142-5, (1983)
全文:HTML全文
摘要
The kinetic barrier against the transport of methyl and ethyl nicotinates across the water-isopropyl myristate interface has been studied as a function of temperature using a rotating diffusion cell. The temperature dependence of the interfacial transfer kinetics has enabled calculation of the thermodynamic parameters for the process. It is evident from the results that, for the transferring solutes considered, the activation energy barrier is enthalpic, because there is a large positive entropy for transfer into the interfacial region.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
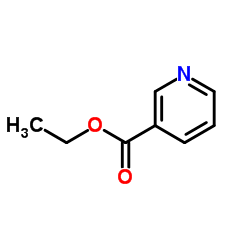 |
烟酸乙酯
CAS:614-18-6 |
C8H9NO2 |
相关文献:
更多...
|
In vitro metabolism and stability of the actinide chelating ...
2015-05-01 [J. Pharm. Sci. 104(5) , 1832-8, (2015)] |
|
Inhibitory effects of niacin and its analogues on induction ...
1987-09-15 [Biochem. Pharmacol. 36(18) , 3015-9, (1987)] |
|
Inhibitory effect of 3-carboethoxypyridine and 3-carbobutoxy...
2007-01-01 [Acta Pol. Pharm. 64(2) , 179-82, (2007)] |
|
Utility of a three-dimensional cultured human skin model as ...
2004-10-01 [Drug Metab. Pharmacokinet. 19(5) , 352-62, (2004)] |
|
Transdermal nicotine suppresses cutaneous inflammation.
1997-07-01 [Arch. Dermatol. 133(7) , 823-5, (1997)] |