Application of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite for in situ preparation of deoxyribonucleoside phosphoramidites and their use in polymer-supported synthesis of oligodeoxyribonucleotides.
J Nielsen, M Taagaard, J E Marugg, J H van Boom, O Dahl
文献索引:Nucleic Acids Res. 14(18) , 7391-403, (1986)
全文:HTML全文
摘要
Deoxyribonucleoside phosphoramidites are prepared in situ from 5'-O,N-protected deoxyribonucleosides and 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite with tetrazole as catalyst, and the solutions applied directly on an automatic solid-phase DNA synthesizer. Using LCAA-CPG support and a cycle time of 12.5 min, oligonucleotides of 16-25 bases are obtained with a DMT-efficiency per cycle of 98.0-99.3%. The crude and fully deblocked products are of a purity comparable to that obtained using purified phosphoramidites. In case of d(G)16 the product was difficult to analyse and a better product was not obtained using doubly protected (O-6 diphenylcarbamoyl) guanine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
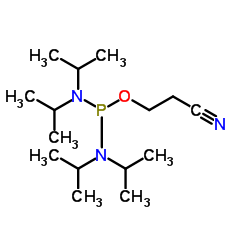 |
双(二异丙基氨基)(2-氰基乙氧基)膦
CAS:102691-36-1 |
C15H32N3OP |
|
2'-fluoro-4'-thioarabino-modified oligonucleotides: conforma...
2007-01-01 [Nucleic Acids Res. 35(5) , 1441-51, (2007)] |
|
DNA adducts of acrolein: site-specific synthesis of an oligo...
2002-05-01 [Chem. Res. Toxicol. 15(5) , 607-13, (2002)] |
|
Synthesis and fluorescence studies of multiple labeled oligo...
2004-01-01 [Bioconjug. Chem. 15 , 638-646, (2004)] |
|
Synthesis of 1,2-diacyl-sn-glycerophosphatidylserine from eg...
1996-05-01 [Lipids 31(5) , 541-6, (1996)] |
|
LNA guanine and 2,6-diaminopurine. Synthesis, characterizati...
2004-05-01 [Bioorg. Med. Chem. 12 , 2385-2396, (2004)] |