Ether lipid synthesis from enantiomeric medium-chain and long-chain O-alkyl-sn-glycerols in Leishmania donovani promastigotes.
H O Herrmann, U Hintze, G Gercken
文献索引:Mol. Biochem. Parasitol. 3(5) , 319-25, (1981)
全文:HTML全文
摘要
A medium-chain O-alkylglycerol, 1-O-[1'-14C]dodecyl-sn-glycerol, has been found to be incorporated into plasmenyl ethanolamine by Leishmania donovani promastigotes as revealed by radio gas-liquid chromatography; however, the ether bond of the administered O-alkyl-glycerol was cleaved extensively as judged from the occurrence of radioactive acyl moieties. The labelling pattern produced by the radioactive 'natural' 1-O-octadecyl-sn-glycerol was similar though the latter served as a slightly better substrate for plasmalogens. Experiments with the enantiomeric 3-O-alkyl-sn-glycerols in comparison revealed that these were poor substrates for plasmalogen synthesis, although they were taken up in identical amounts and cleaved even to a higher extent. Therefore, we conclude the 1-0-alkyl-sn-glycerols were utilized directly for plasmenyl ethanolamine synthesis. The incorporation of the dodecyl residue into plasmenyl ethanolamine did not affect the multiplication and shape of cells.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
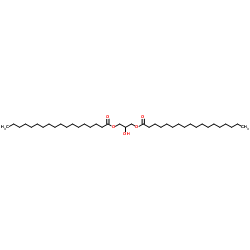 |
1,3-二硬脂酸甘油酯
CAS:504-40-5 |
C39H76O5 |
|
Mixed triglyceride breath test: a noninvasive test of pancre...
1989-04-01 [Gastroenterology 96(4) , 1126-34, (1989)] |
|
Structural analogies between protein kinase C activators.
1985-03-29 [Biochem. Biophys. Res. Commun. 127(3) , 969-76, (1985)] |
|
Synthesis and analysis of symmetrical and nonsymmetrical dis...
2003-03-26 [J. Agric. Food Chem. 51(7) , 2096-9, (2003)] |
|
Phase and surface properties of lipid bilayers containing ne...
1999-03-01 [Arch. Biochem. Biophys. 363(1) , 81-90, (1999)] |