AE2 anion exchanger polypeptide is a homooligomer in pig gastric membranes: a chemical cross-linking study.
A S Zolotarev, B E Shmukler, S L Alper
文献索引:Biochemistry 38(26) , 8521-31, (1999)
全文:HTML全文
摘要
Although considerable information is available on the oligomeric states of the AE1 (band 3) anion exchanger, little is known about the physiological state of the polypeptides encoded by the nonerythroid AE genes, AE2 and AE3. We have previously characterized the proteolytic susceptibility of native pig gastric AE2. In the course of studies in which pig gastric membranes were treated with the AE2 transport antagonist, DIDS, we noted evidence for cross-linking of AE2 proteolytic fragments to higher-order oligomeric forms. We have characterized the ability of DIDS and of selected N-hydroxysuccinimide cross-linking agents to increase the proportion of SDS-resistant oligomers of pig gastric AE2 and its proteolytic fragments. Cross-linking exhibited time and concentration dependence. N-Terminal protein sequencing proved that DIDS treatment created AE2 homodimers. Putative homotetramers were also observed. Protomers were cross-linked via residues within the C-terminal 40 kDa of AE2. Prior proteolytic cleavage of AE2 in membranes resulted in decreased yield of subsequently cross-linked products. AE2 cross-linking could not be detected in membranes pretreated by hypotonic wash and freeze-thaw. The results are interpreted in light of the deduced amino acid sequence of the transmembrane domain of pig AE2.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
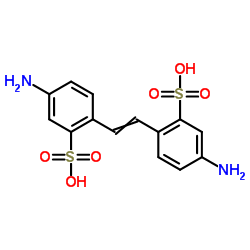 |
4,4'-二氨基二苯乙烯-2,2'-二磺酸
CAS:81-11-8 |
C14H14N2O6S2 |
|
Glutathione- and thioredoxin-related enzymes are modulated b...
2007-10-01 [Biol. Chem. 388(10) , 1069-81, (2007)] |
|
Human SLC4A11-C functions as a DIDS-stimulatable H⁺(OH⁻) per...
2015-01-15 [Am. J. Physiol. Cell Physiol. 308(2) , C176-88, (2015)] |
|
Uterotropic action in rats of amsonic acid and three of its ...
1992-05-01 [J. Toxicol. Environ. Health A 36(1) , 13-25, (1992)] |
|
Evaluation of the disodium salt of 4,4'-diamino-2,2'-stilben...
1996-06-07 [J. Toxicol. Environ. Health A 48(2) , 141-9, (1996)] |
|
Comparison of -nitro versus -amino 4,4'-substituents of disu...
1994-11-23 [Mol. Cell Biochem. 140(2) , 137-46, (1994)] |