The glycoprotein nature of pig kidney diamine oxidase. Role of disulphide groups and arginine residues in the concanavalin A-diamine oxidase interaction.
M A Shah, R Ali
文献索引:Biochem. J. 253(1) , 103-7, (1988)
全文:HTML全文
摘要
Pig kidney diamine oxidase (DAO) was found to contain 5% (w/w) natural hexose, 3.25% glucosamine, 2.61% N-acetylglucosamine and 0.25% N-acetylneuraminic acid. The enzyme exhibited strong affinity towards concanavalin A (Con A) with a stoichiometry of 1:4.6. The kinetics of interaction approached an apparent first-order rate, with a rate constant (Kapp.) value of 1.5 x 10(-2) min-1. The enzyme reduced with dithiothreitol followed by alkylation with iodoacetamide showed an increase in the stoichiometry of the Con A-DAO interaction. Similarly arginine modification by phenylglyoxal caused decreased affinity, with an altered Kapp. value of 9.09 x 10(-3) min-1. The results suggest that, besides the carbohydrate content, the protein moiety of the enzyme also plays a significant role in the Con A-DAO interaction.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
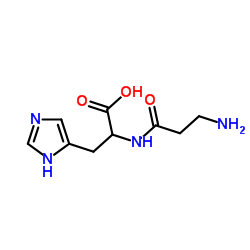 |
二胺氧化酶
CAS:9001-53-0 |
C9H14N4O3 |
|
Diamine oxidase from pig kidney: new purification method and...
1982-01-01 [Prep. Biochem. 12(1) , 11-28, (1982)] |
|
A luminescence-based test for determining ornithine decarbox...
2000-12-15 [Anal. Biochem. 287 , 299-302, (2000)] |
|
N-linked oligosaccharide structures in the diamine oxidase f...
2000-01-12 [Carbohydr. Res. 323 , 111-25, (2000)] |
|
Diamine oxidase and catalase are expressed in the same cells...
1999-04-01 [Inflamm. Res. 48 , s81-2, (1999)] |
|
Evolution of a constitutional dynamic library driven by self...
2010-04-26 [Chemistry 16(16) , 4903-10, (2010)] |