Enantiomer separation of mandelates and their analogs on cyclodextrin derivative chiral stationary phases by capillary GC.
M Y Nie, L M Zhou, Q H Wang, D Q Zhu
文献索引:Anal. Sci. 17(10) , 1183-7, (2001)
全文:HTML全文
摘要
Enantiomer separation of mandelates and their analogs, which are important intermediates in asymmetric synthetic and pharmaceutical chemistry, was investigated by capillary gas chromatography using different cyclodextrin derivative chiral stationary phases (CD CSPs). The used cyclodextrin derivatives included permethylated beta-CD (PMBCD), permethylated gamma-CD, heptakis(2,6-di-O-butyl-3-O-butyryl)-beta-CD, heptakis(2,6-di-O-pentyl-3-O-acetyl)-beta-CD and heptakis(2,6-di-O-nonyl-3-O-trifluoroacetyl)-beta-CD (DNTBCD), respectively. Among all the CSPs used, PMBCD and DNTBCD exhibited the broadest and best enantioselectivity for all the racemates investigated. Some thermodynamic parameters were evaluated and an enthalpy-entropy compensation effect was observed in enantiomer separation processes of mandelates and their analogs. Based on thermodynamic data and molecular mechanics calculations, the chiral recognition mechanism of mandelate derivatives on CD CSPs is discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
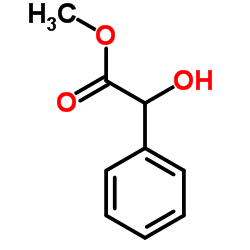 |
DL-扁桃酸甲酯
CAS:4358-87-6 |
C9H10O3 |
|
Hydrolysis of triacetin catalyzed by immobilized lipases: Ef...
2011-01-01 [Enzyme Microb. Technol. 48 , 510-517, (2011)] |
|
Esters of mandelic acid as substrates for (S)-mandelate dehy...
2004-02-24 [Biochemistry 43(7) , 1883-90, (2004)] |
|
Generation of the enol of methyl mandelate by flash photolys...
2000-02-25 [J. Org. Chem. 65(4) , 1175-80, (2000)] |
|
Theoretical study on chiral recognition mechanism of methyl ...
2012-02-01 [J. Mol. Model. 18(2) , 803-13, (2012)] |
|
Calculations of the energy distribution from perturbation pe...
2008-01-01 [J. Chromatogr. A. 1203(2) , 177-84, (2008)] |