Development and validation of an HPLC method for the determination of dibenzoylmethane in rat plasma and its application to the pharmacokinetic study.
Guoxiang Shen, Jin-Liern Hong, Ah-Ng Tony Kong
文献索引:J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 852(1-2) , 56-61, (2007)
全文:HTML全文
摘要
A highly sensitive and simple high-performance liquid chromatographic (HPLC) assay has been developed and validated for the quantification of dibenzoylmethane (DBM) in rat plasma. DBM and internal standard (I.S.) 1-(5-chloro-2-hydroxy-4-methylphenyl)-3-phenyl-1,3-propanedione (CHMPP) were extracted from rat plasma by ethyl acetate/methanol (95:5, v/v) and analyzed using reverse-phase gradient elution with a Phenomenex Gemini C18 5-mum column. A gradient of mobile phase (mobile phase A: water/methanol (80:20, v/v) with 0.1% TFA and mobile phase B: acetonitrile with 0.1% TFA) at a flow rate of 0.2 mL/min, and ultraviolet (UV) detection at 335 nm were utilized. The lower limit of quantification (LLOQ) using 50 microL rat plasma was 0.05 microg/mL. The calibration curve was linear over a concentration range of 0.05-20 microg/mL. The mean recoveries were 80.6+/-5.7, 83.4+/-1.6 and 77.1+/-3.4% with quality control (QC) level of 0.05, 1 and 20 microg/mL, respectively. Intra- and inter-day assay accuracy and precision fulfilled US FDA guidance for industry bioanalytical method validation. Stability studies showed that DBM was stable in rat plasma after 4h incubation at room temperature, one month storage at -80 degrees C and three freeze/thaw cycles, as well as in reconstitute buffer for 48 h at 4 degrees C. The utility of the assay was confirmed by the successful analysis of plasma samples from DBM pharmacokinetics studies in the rats after oral and intravenous administrations.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
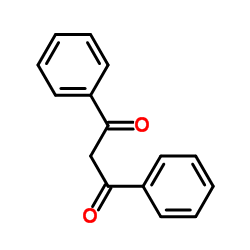 |
二苯甲酰甲烷
CAS:120-46-7 |
C15H12O2 |
|
Identification and characterization of novel benzil (dipheny...
2005-04-21 [J. Med. Chem. 48 , 2906-15, (2005)] |
|
A glimpse of the inner workings of the templated site.
2009-01-08 [Chem. Commun. (Camb.) (2) , 165-7, (2009)] |
|
Combining ligand-based pharmacophore modeling, quantitative ...
2008-10-23 [J. Med. Chem. 51 , 6478-94, (2008)] |
|
Faster Synthesis of Beta-Diketonate Ternary Europium Complex...
2015-01-01 [PLoS ONE 10 , e0143998, (2015)] |
|
Dietary phytochemicals induce p53- and caspase-independent c...
2011-11-01 [Int. J. Dev. Neurosci. 29(7) , 701-10, (2011)] |