Activity of Subtilisin Carlsberg in macromolecular crowding.
Ajay Kumar Shaw, Samir Kumar Pal
文献索引:J. Photochem. Photobiol. B, Biol. 86(3) , 199-206, (2007)
全文:HTML全文
摘要
Enzymatic activity of a proteolytic enzyme Subtilisin Carlsberg (SC) in anionic sodium dodecyl sulfate (SDS) micellar medium has been explored and found to be retarded compared to that in bulk buffer. Circular dichroism (CD) study reveals that SDS, which is a potential protein denaturant, has an insignificant denaturation effect on SC. The structural integrity of the protein offers an opportunity to study the functionality of the enzyme SC in a macromolecular crowding of micelles. Dynamic light scattering (DLS) data indicates no sandwich-like micelle-SC complex formation ruling out the possibility of interaction of the enzyme with the hydrophobic core of the micelle. However, steady state and time resolved emission studies on specific and nonspecific fluorescent probes indicate the proximity effect at the surface of the enzyme due to macromolecular crowding of the micelles. The agreement of retarded enzymatic activity in the micellar crowd with a theoretical model ascribed to the facts that substrates are compartmentalized in the micelles and enzyme interacts with the micelle through stern layer.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
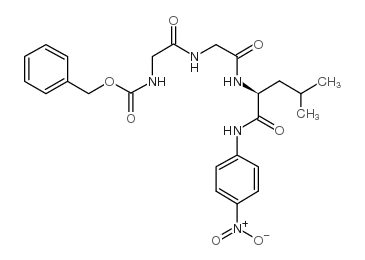 |
Z-GLY-GLY-LEU-PNA
CAS:53046-98-3 |
C24H29N5O7 |
|
A new chromogenic substrate for subtilisin.
1974-12-01 [Anal. Biochem. 62 , 371-376, (1974)] |
|
Degradation of bradykinin by isolated neutral endopeptidases...
1979-09-12 [Biochem. Biophys. Res. Commun. 90 , 1, (1979)] |
|
Evidence that pituitary cation-sensitive neutral endopeptida...
1983-03-01 [J. Neurochem. 40 , 842-849, (1983)] |
|
A synthetic endopeptidase substrate hydrolyzed by the bovine...
1984-05-01 [Exp. Eye Res. 38(5) , 477-83, (1984)] |
|
Technical note: quantification of multicatalytic proteinase ...
1993-12-01 [J. Anim. Sci. 71(12) , 3301-6, (1993)] |